-
Call Now
1800-102-2727
Born Haber Cycle
Born Haber Cycle is a process utilised to assess and investigate the energies in the reactions. Particularly it aids in illustrating the generation of ionic compounds from distinct elements. Furthermore, lattice energy is widely calculated using the Born Haber cycle. Also, it comprises a range of phases and processes like electron affinity, formation of what, dissociation energy, ionisation energy and sublimation energy. Thus, the cycle is helpful in stepwise observing the overall processing of a reaction.
Table of Contents
- What is the Born Haber Cycle?
- What is Lattice Energy?
- Concept of Born Haber Cycle
- What is Hess Law?
- Understanding Basic Concepts
- Born Haber Cycle Equation
- How to Apply the Born Haber Equation?
- Practice Problems
- Frequently Asked Questions
What is the Born Haber Cycle?
The born-haber cycle is an iterative process of enthalpy change. It forms solid crystalline ionic compounds from atoms of the elements in their standard state with a net enthalpy zero. The born haber cycle is a key process to calculate the lattice energy, which cannot be measured otherwise.
The cycle mainly focuses on generating ionic compounds from group 1 and 2 metals when they react with halogen or non-metallic elements like oxygen.
What is Lattice Energy?
Lattice energy is a potential energy required to dissociate and convert an ionic solid into its constituent atoms into ions in the gaseous phase. Though the lattice energy reaction is endothermic, the amount of lattice energy would always be positive. In other words, The energy produced when ions present in the gaseous form are linked together to form an ionic solid is known as lattice energy. As per this definition, the procedure would always be exothermic, thus resulting in a negative value of lattice energy. Often the value is expressed in kilo Joule per molecule unit, i.e., kJ/mol.
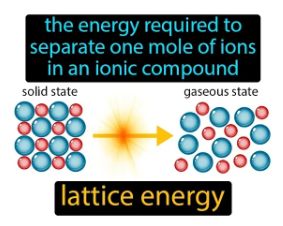
Image: Lattice Energy
Concept of Born Haber Cycle
The interactions between electropositive metals with electronegative non-metals produce ionic solids. The reaction between chalcogen or other halogen family elements with alkali or alkaline earth metals forms crystalline ionic solid compounds. Ionic compounds must have identical physical properties as the electrostatic force of interaction between positive and negative charge is stable. Although, the variability has been observed in other physical properties like water solubility and stability difference.
This variation is due to the difference in enthalpy-like Lattice energy between the two ionic solids. The compound’s anions and cations are bound together in fixed positions by lattice energy in crystalline solids. When the energy is released from one mole of an ionic solid into its gaseous ions or the release of energy required to transition into its gaseous ions is created from one mole of a solid ionic compound is lattice energy. Hess law is used to measure the lattice energy appropriately.
What is Hess Law?
According to Hess' law, also referred to as Hess's law of constant heat summation, "at a constant temperature, heat energy changes underlying a chemical reaction shall remain unchanged, regardless of how the reactants react to produce the product."
Hess' law is centred on the state function nature of enthalpy and the first law of thermodynamics. Therefore, the enthalpy of the molecules in the reactant and product is constant and unaffected by their source and process of production.
Hess's law enables us to determine the overall difference in enthalpy by only adding up the changes at every stage up till the formation of the final product. The individual step equations must balance, and each step should take place at the same temperature.
Understanding of Basic Concepts
It is important to understand certain concepts before applying the Born Haber cycle to calculate the lattice energy of an ionic solid.
1. Ionisation Energy
Ionisation energy is the positive energy required to remove an electron from a neutral ion or atom. The ionisation energy increases from left to right and drops from top to bottom in a periodic table. Certain exceptions are applicable for those related to half or completely-filled orbitals.
2. Sublimation Energy
The amount of energy required for a phase transition directly from solid to gaseous while avoiding the liquid phase is called sublimation energy. It has a positive value due to being input energy and is sometimes called atomisation energy. When a compound is generated from its constituent components, the change in energy is called the heat of formation. It can be negative or positive, completely relying on the atoms involved and their interactions.
3. Dissociation Energy
Dissociation energy is the amount of energy required to capture a chemical. Generally, the dissociation of chemicals is an endothermic reaction, thus needing an energy input to proceed with the process. Therefore, every energy shaft is good, and the involved atom’s electronegativity illustrates the size of the energy.
4. Electron Affinity
When an electron is introduced to a neutral ion or atom, energy is created called electron affinity. The energy emitted is negative, but most tables express a positive value per the electron affinity definition. Thus, subtracting the electron affinity instead of adding it while calculating lattice energy is important. Also, electron affinity increases from left to right and drops from top to bottom across the periodic table.
Born Haber Cycle Equation
Born Haber cycle equation or formula can be written as the following.

Image: Born Haber Cycle Equation
The fundamental equation includes electron affinity. However, observing either its exothermic reaction (release of energy) or endothermic reaction (energy absorbance) for each electron affinity is important. If the energy is released, the value must be negative and vice versa. By rearranging the equation, we get the following equation.
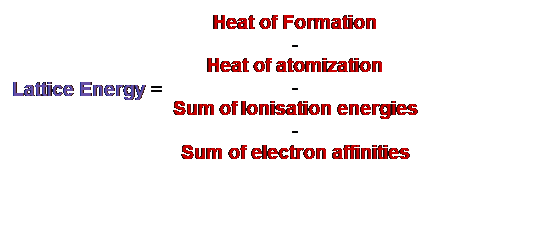
Image: Rearranged Formula of Born Haber Cycle
How to Apply the Born Haber Equation?
There are certain steps to apply the Born Haber Cycle. They are as follows:
Step 1
Elaborate the metal and non-metal energies as elemental forms. Then subtract the ionic solids heat of formation from mixing these elements in the right proportion. We will gain the energy of the ionic solid.
Step 2
The reacting components must be in the gaseous phase for the Born Haber cycle. To convert an element to its gaseous form, add the enthalpy change and proceed with the same with other elements.
Step 3
Metals exhibit one atom and thus do not require dissociation energy. At the same time, non-metals exhibit in polyatomic form and need to be dissociated. For example, chlorine has two atoms, i.e., Cl2 , the value that must be changed to 2Cl atoms, which is gained in Step 2.
Step 4
To be in ionic solids, both metals and nonmetals are altered now to be in ionic forms. The value gained in Step 3 is added to the ionisation energy to achieve the transformation into ionic form. Furthermore, the electron affinity is deducted from the preexisting value since adding electrons releases energy.
Step 5
Proceeding with the reaction, metal and non-metals will now be mixed to form an ionic solid, releasing Lattice energy. The amount of the lattice energy can be obtained by subtracting the value obtained in Step 4 from Step 1.

Image: Equation of Born Haber Cycle with Example Values
Practice Problems
Q1. Born Haber cycle is used to calculate
A. Ionic compounds
B. Energy in a chemical reaction
C. Non-metallic elements
D. Metallic elements
Answer: b. Energy in a chemical reaction. Born Haber Cycle is a procedure to determine the energy in a chemical reaction.
Q2. The Born Haber cycle includes processes like
A. Dissociation energy
B. Ionisation energy
C. Electron affinity
D. All of the above
Answer: D. All of the above. The Born Haber cycle involves distinct processes and phases like dissociation energy, ionisation energy, electron affinity, formation of heat, and sublimation energy.
Q3. Lattice energy involves the breakdown and transformation of ionic solids into
A. Liquid form
B. Gaseous form
C. Solid form
D. None of the above
Answer: B. Gaseous form. Lattice energy is important to dissociate ionic solids and convert their constituents into gaseous ions.
Frequently Asked Questions
Q1. Do covalent compounds exhibit lattice energy?
Answer: No. Covalent compounds do not possess lattice energy as they are not composed of ions.
Q2. Why do ionic compounds exhibit more stability?
Answer: Ionic compounds exhibit more stability because their solid forms consist of lattice energy.
Q3. What does the negative charge of the lattice enthalpy state?
Answer: The negative charge of the lattice enthalpy states that there is a stronger ionic bond in an ionic compound.


