-
Call Now
1800-102-2727
Beta Elimination: Definition, Mechanism, Types-E1, E2, E1cB Elimination, Practice problems, FAQs
Imagine a beauty pageant contest in which contestants from various countries participated. In the finale, contestants from five countries are left and are made to stand in a line on a stage. In the second last round contestants from the Philippines and France get eliminated such that those from Venezuela, South Africa and India, come closer. The ultimate aim of the contest is to filter the best suitable candidate.

Similarly, in some organic reactions, atoms from two adjacent carbon atoms are eliminated to form a product containing a stronger -bond.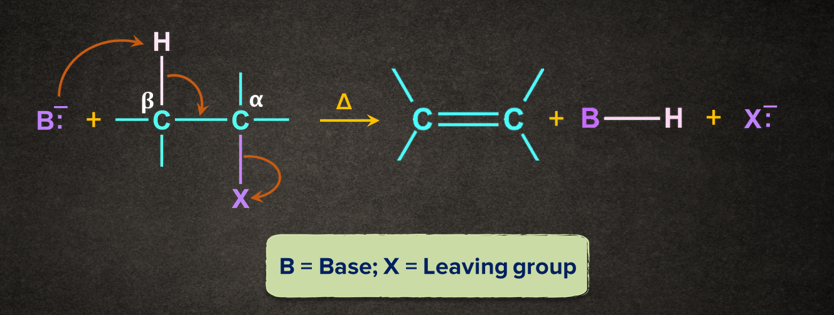
Depending upon the fashion/style of how two atoms are eliminated, they are of three types, E1, E2, E1cB.
Table of content
- What is -Elimination?
- Mechanism of -Elimination
- Types of 𝛃-elimination
- E1 Elimination
- E2 Elimination
- E1cB Elimination
- Practice Problems
- Frequently asked Questions(FAQs)
-Elimination
In -elimination, two atoms/substituents are removed from the adjacent atoms ( and positions) to form a new multiple bond. It is also known as 1,2- elimination.
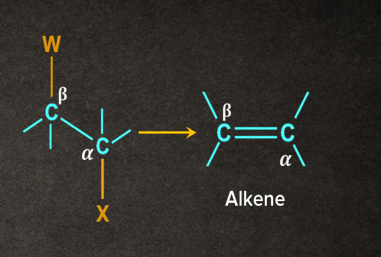
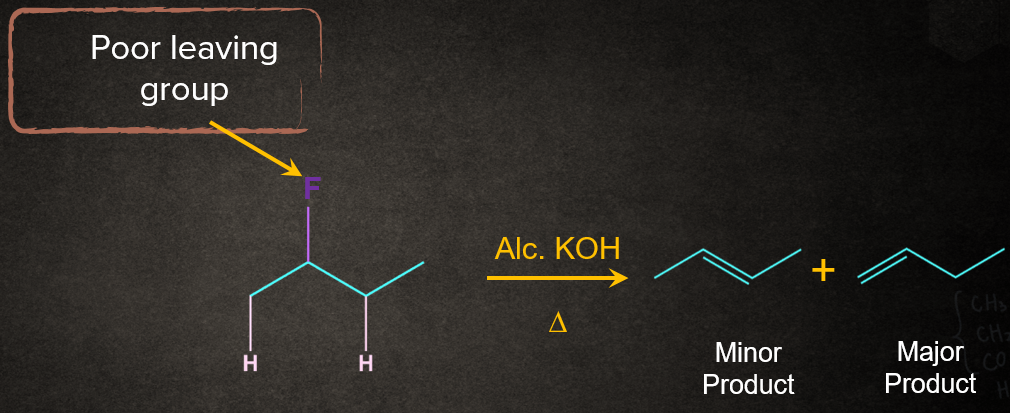
For example, when a haloalkane is made to react with a base, B-, in the presence of heat (), the elimination of the halogen (-X) from the -carbon and proton (-H+) from the -carbon takes place to give the corresponding alkene.
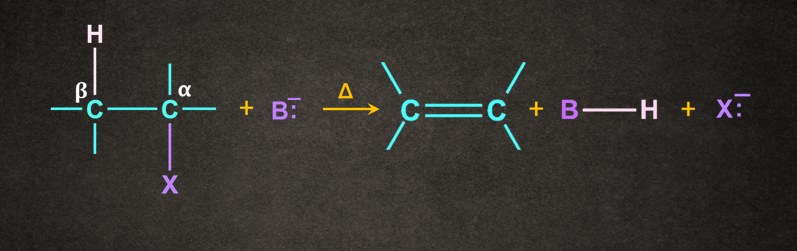
Mechanism of -elimination
Generally, the base ( B-) abstracts a proton ( H+) from the -carbon followed by the removal of halide ion(X-) from the -carbon. This can be understood with the help of the mechanism given below.
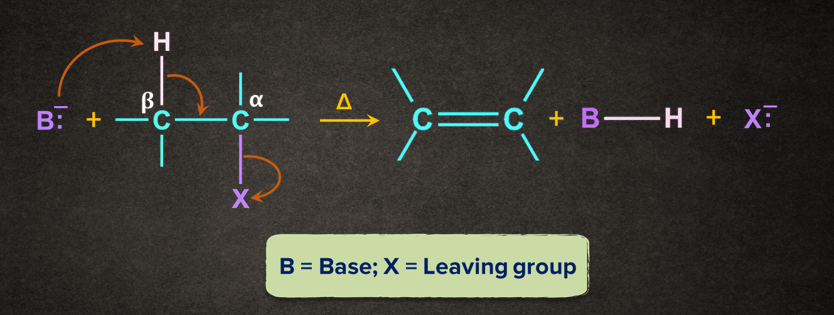
One of the important examples of -Elimination is dehydrohalogenation. In dehydrohalogenation, hydrogen from the 𝛃-C and a halogen from the 𝝰-C get eliminated when heated with an alcoholic solution of KOH.
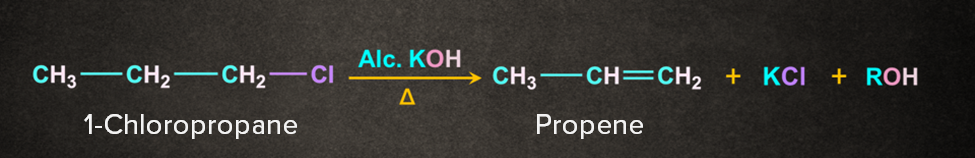
Types of 𝛃-elimination
Based on the fact that either abstraction of a proton or the removal of halogen is the first step or there will be simultaneous removal, elimination reactions can be classified into three types.
1. E1 elimination
2. E2 elimination
3. E1cB elimination
E1 Elimination
In E1 elimination, the leaving group leaves the substrate first to form a carbocation intermediate. Then, the proton abstraction takes place to form an alkene. E1 elimination can be explained in detail by the following reactions.
Example- Dehydrohalogenation of an alkyl halide in the presence of water.
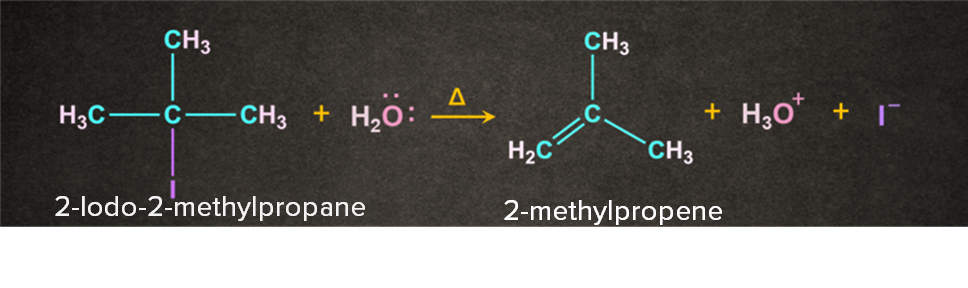
Reactions That Follow E1 Mechanism
- Dehydrohalogenation of alkyl halide
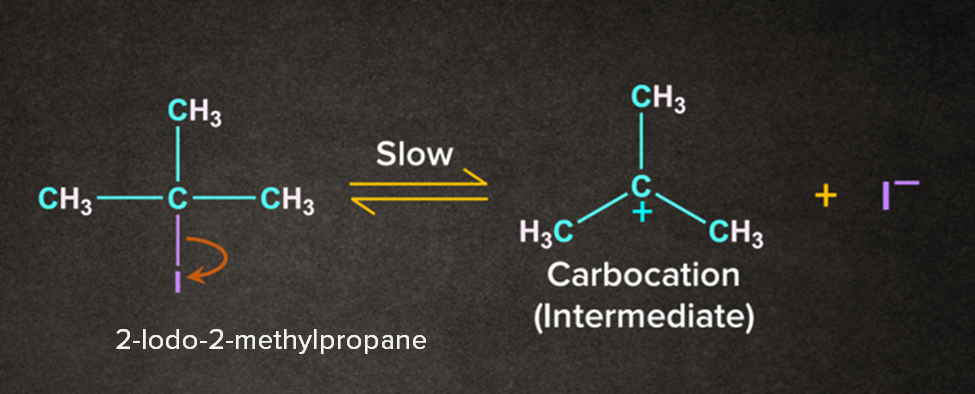
As the name dehydrohalogenation suggests, halogen and hydrogen are removed. The reaction occurs in the presence of a weak base like H2O. It is a two-step process. Let's consider a reaction of 2-Iodo-2-methylpropane with H2O in the presence of heat. The reaction occurs in two steps as follows:
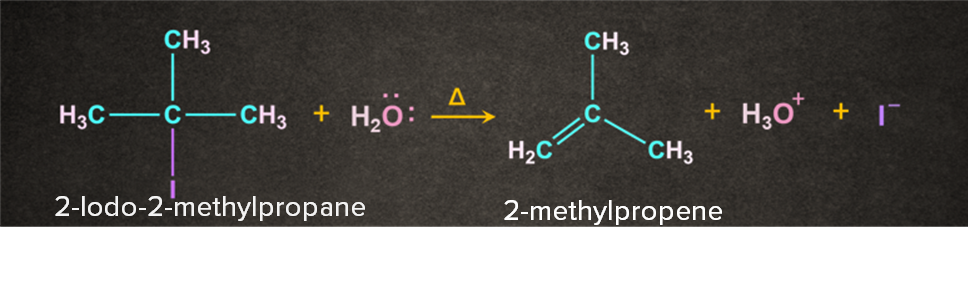
Step 1: Formation of carbocation
-I being electronegative leaves as I- to form a tertiary carbocation.
Step 2: Attack of base
H2O being a weak base abstract the H+ from the -carbon to give 2-methylpropene.
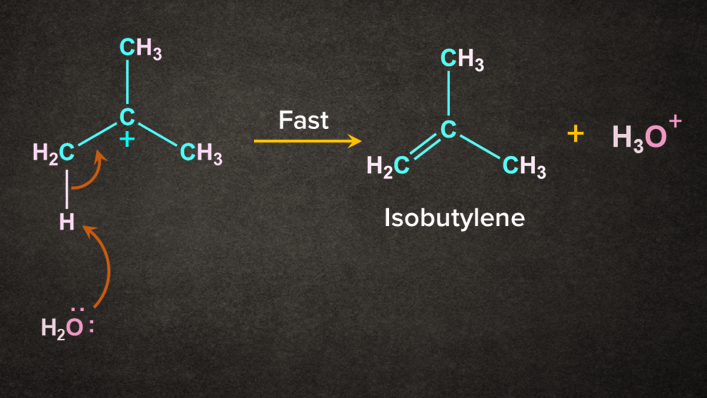
- Dehydration of Alcohol
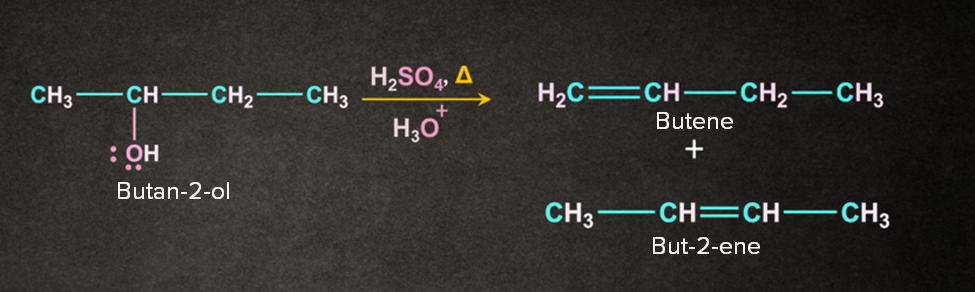
Let us consider the dehydration reaction of butan-2-ol.
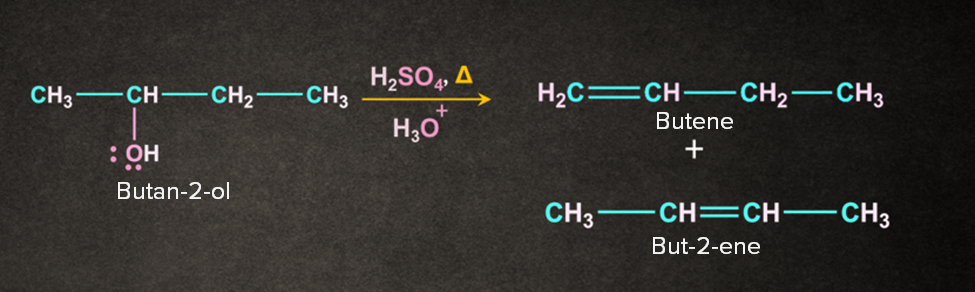
The reaction occurs in three steps.
Step 1: Protonation of alcohol
Oxygen being electron rich accepts the H+ to give protonated alcohol.
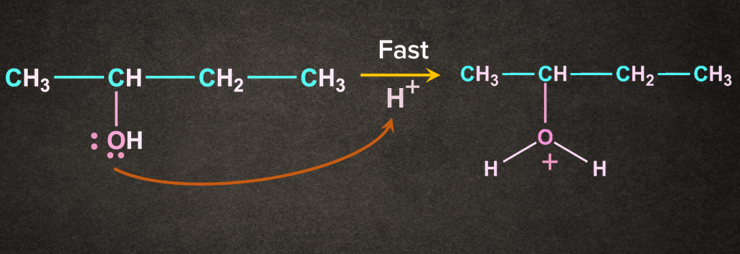
Step 2: Loss of leaving group
Protonated alcohol removes a water molecule to give carbocation. The carbocation formed here is a stable secondary carbocation.
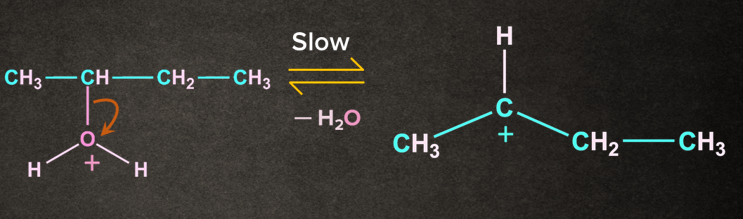
Step 3: Deprotonation to form alkene
There is the removal of -hydrogen to give an alkene. There are two types of -hydrogen available i.e. a and b.
Case 1: Removal of proton ‘a’ gives but-1-ene
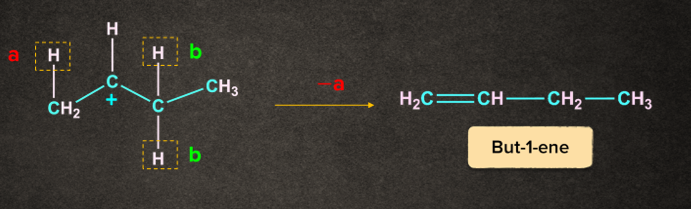
Case 2: Removal of proton ‘b’ gives but-2-ene.
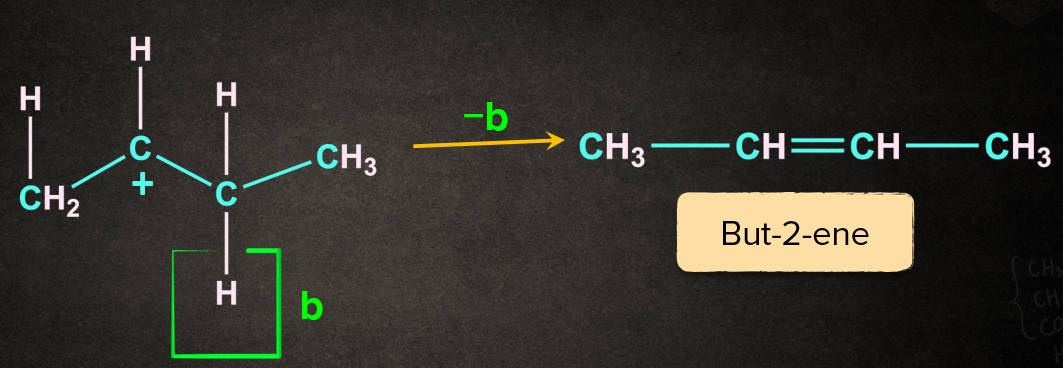
The reaction results in the formation of two products. The major product is decided with the help of Saytzeff's rule. According to this rule, “The most substituted alkene is the major product. Generally, it is the most preferred one.” But-2-ene being the most substituted of the two will be the major product.
Rate of the Reaction in E1 Mechanism
The formation of the carbocation is the rate-determining step in the E1 elimination reaction.
|
Rate of reaction ∝ [R-X]1 [B]0 |
Thus, E1 elimination is a first-order reaction, i.e., a unimolecular reaction. The more the stability of the carbocation intermediate, the more the rate of the reaction. The increasing order of stabilities of carbocations is:
|
Primary carbocation < Secondary carbocation < Tertiary carbocation |
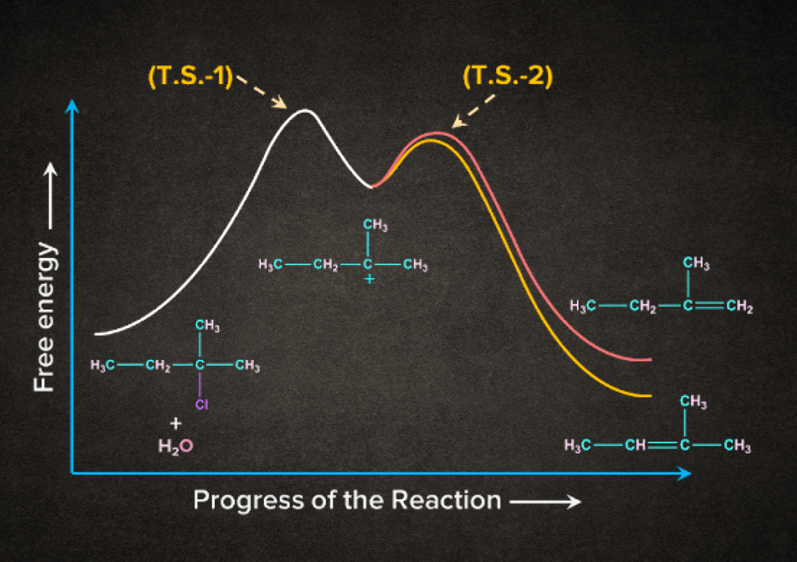
Energy Profile Diagram for E1 Elimination Reaction
Let us take the example of 2-chloro-2-methylbutane. It undergoes E1 elimination such that two products are obtained, 2-methyl-2-butene and 2-methyl-1-butene.
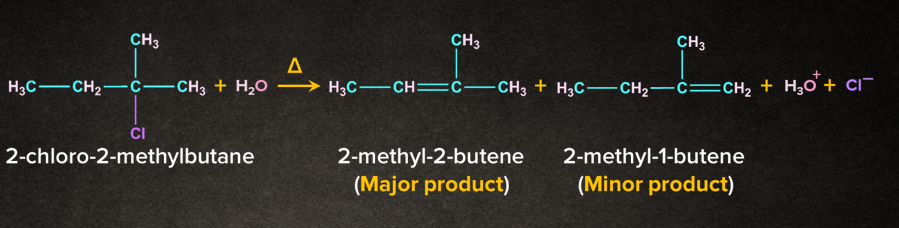
According to the Saytzeff rule, 2-methyl-2-butene will be the major product. In the E1 reaction of 2-chloro-2-methylbutane, the first step involves the formation of carbocation intermediate via T.S. -1 (Transition State - 1). The removal of -hydrogen leads to the formation of two products. 2-methyl-2-butene being the stable alkene will have less energy than the unstable alkene 2-methyl-1-butene.
Note:
|
Stability ∝1Energy |
- The energy of T.S. -1 is greater than the energy of T.S. -2 . This is because in T.S. -1, the reaction is moving towards a less stable and reactive carbocation, but in T.S. -2, The reaction is moving closer to the formation of stable alkene.
Stereochemistry of E1 Mechanism
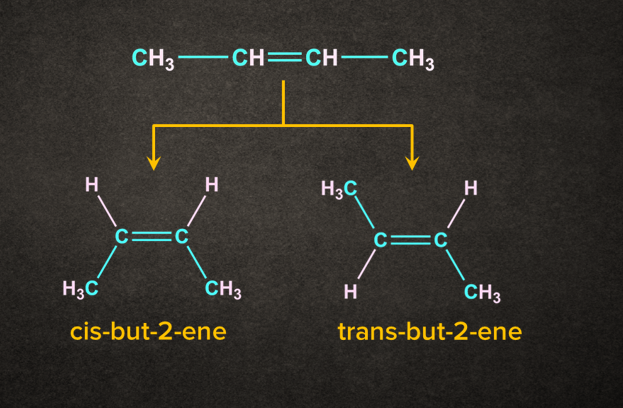
According to the Saytzeff rule, but-2-ene will be the major product. But-2-ene exits in two isomeric forms, that is trans and cis. The trans isomer of but-2-ene would be more stable than the cis isomer. So, trans-but-2-ene would be the overall major product.
E2 Reaction
Generally, two groups/atoms depart simultaneously from adjacent carbons along with the proton being abstracted by a base.
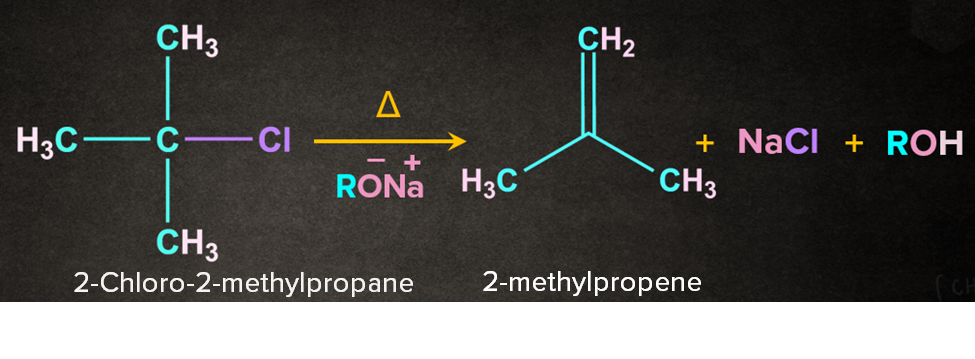
Example- Dehydrohalogenation of alkyl halide in presence of base, RONa.
Reactions That Follow E2 Mechanism
- Dehydrohalogenation of alkyl halide
As the name dehydrohalogenation suggests, halogen and hydrogen are removed. The reaction occurs in the presence of a strong base like RO-Na+. E2 reaction occurs in one step through a transition state. Let's consider a reaction of 2-chloro-2-methylpropane with RO-Na+ in the presence of heat. It is a one-step process.
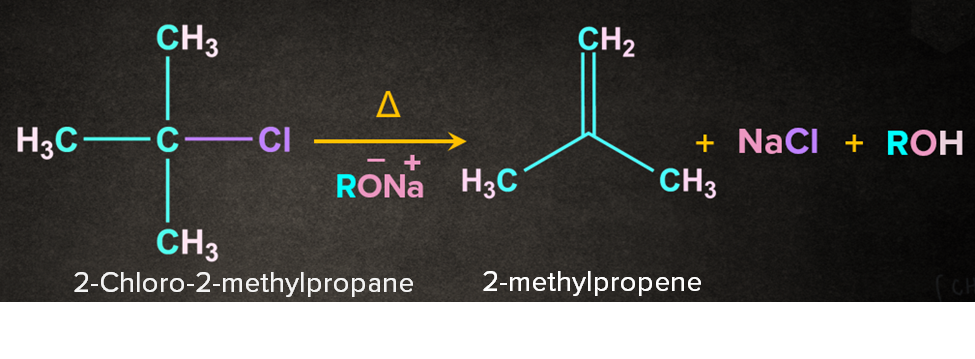
Firstly, RO- abstracts a proton from the -carbon followed by the simultaneous removal of Cl- to give 2-methylpropene. During the abstraction of the proton by the base and the removal of the leaving group, a transition state is formed. A partial double-bond character is observed in the transition state.
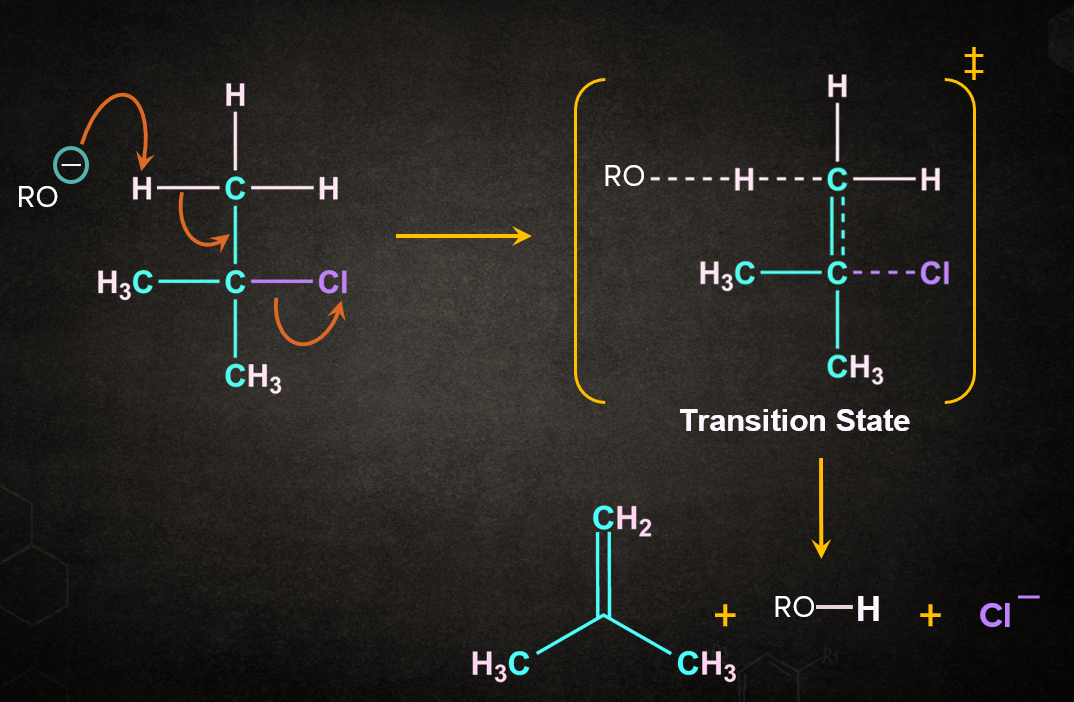
E2 reaction is a concerted reaction because the bond formation and the bond-breaking steps take place simultaneously. A concerted reaction is one in which the abstraction of a proton by a base and the departure of the leaving group occur simultaneously.
Rate of the Reaction in E2 Mechanism
|
Rate of reaction ∝ [R-X]1 [Base]1 |
As the E2 mechanism is a single-step reaction hence, the rate of the reaction depends on the concentration of both the molecules involved. Thus, E2 elimination is a second-order reaction i.e. bimolecular reaction. The stability of alkene governs the rate of E2 reaction.
Example: Order of stabilities of alkenes will be:
CH2=CH2 < CH2=CH-CH3 < CH3-CH=CH-CH3
Greater the stability of the alkene formed, more will be the rate of reaction.
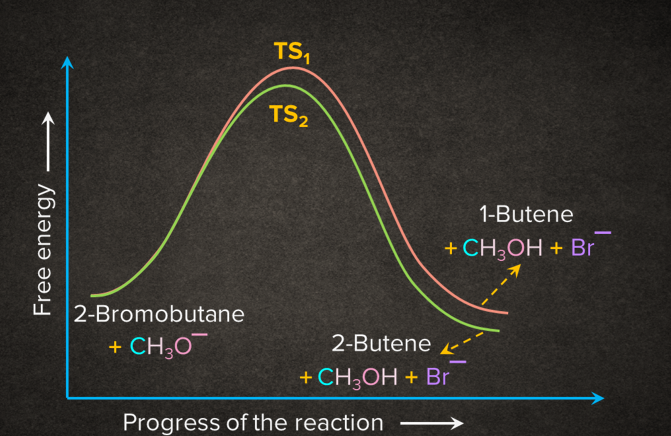
Energy Profile Diagram for E2 Elimination Reaction
Let’s take the example of 2-bromobutane. It undergoes E2 elimination in the presence of methoxide ion such that two products are obtained, 2-butene and 1-butene.
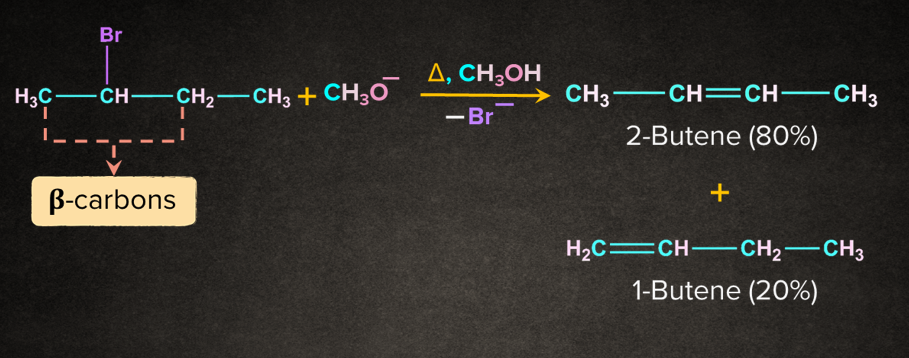
According to the Saytzeff rule, 2-butene will be the major product. In the E2 reaction of 2-bromobutane, removal of bromide and -hydrogen takes place simultaneously. The removal of -hydrogen leads to the formation of two products. 2-butene being the stable alkene will have lesser energy than the relatively unstable alkene 1-butene.
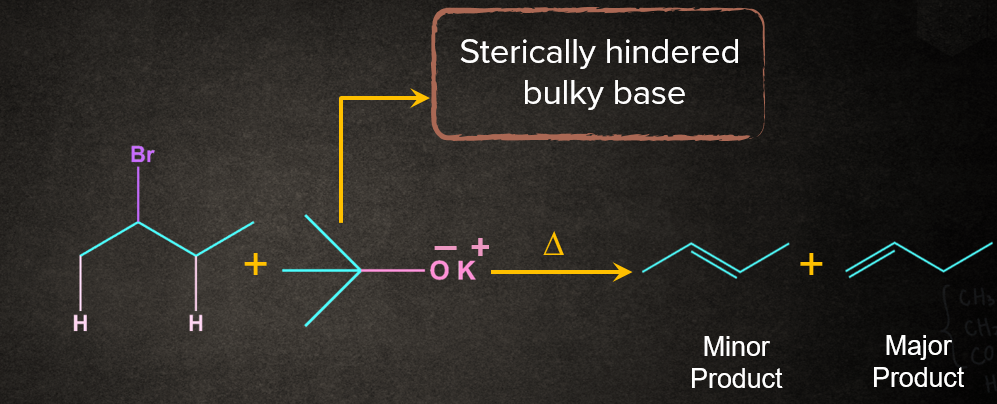
If the base is bulky or when the leaving group in E2 is poor, Hofmann rule is followed. It is just the opposite of the Saytzeff rule. According to the Hofmann rule, the least substituted alkene will be formed as the major product.
Let’s understand the two cases where the Hofmann rule will be followed.
Case 1: When the base is bulky
Case 2: When the leaving group in E2 is poor
Stereochemistry of E2 Mechanism
|
E2 reaction is an anti-elimination reaction. |
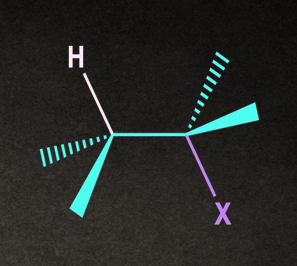
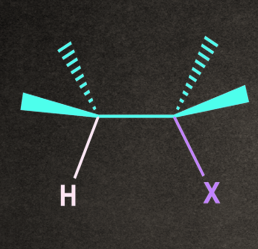
The transition state of E2 consists of an H atom and a leaving group on the substrate aligned in a plane. The alignment can be of two types: Anti-planar and syn-planar.
- Anti-planar: The H and X atoms are oriented on the opposite sides in the plane of the molecule.
- Syn-planar: The H and X atoms are oriented on the same side in the plane of the molecule.
Based on these two ways in which the C-H and C-X bonds align, elimination reactions can be of two types.
- Syn-elimination: If the substituents are removed from the same side of the C-C bond, the reaction is known as syn-elimination.
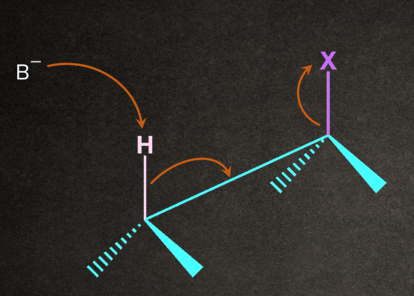
- Anti-elimination: If the substituents are removed from the opposite sides of the C-C bond, the reaction is known as anti-elimination.
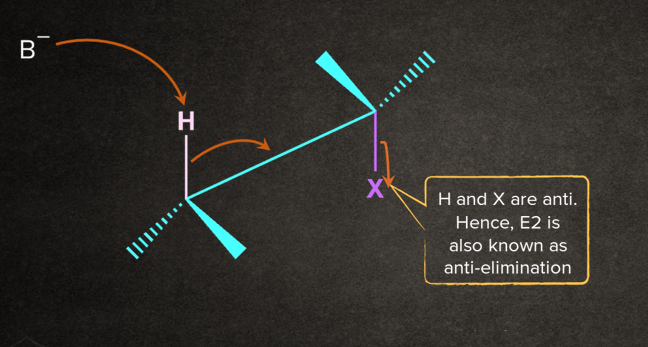
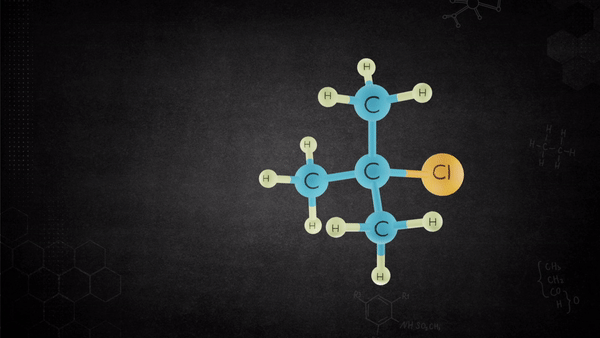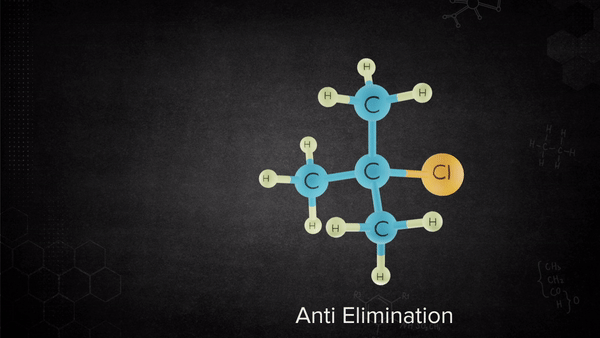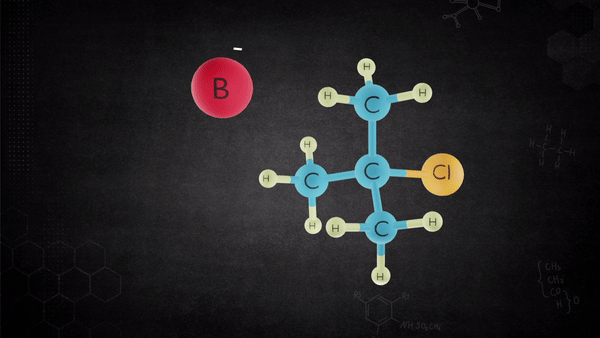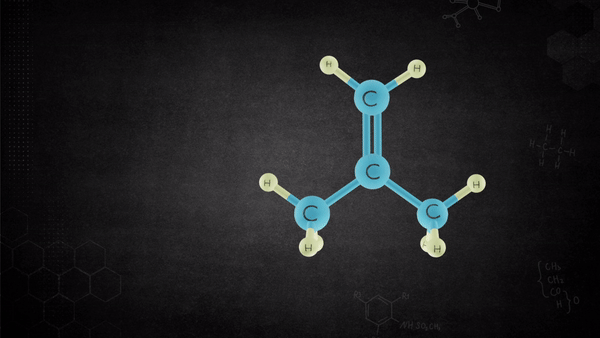
The base removes the proton from the alkyl halide that is oriented anti to the leaving group, and leaving group leaves-all in one concerted step.
E1cB Elimination
In E1cB, E stands for elimination, 1 for unimolecular, and cB for conjugate base. In E1cB, the proton is abstracted to form the conjugate base. The anion that results is stable enough to exist because it can
be delocalised on to the electron-withdrawing group. Although the anion is stabilized by the electron-withdrawing group, it still prefers to lose a leaving group and become an alkene.
Example:
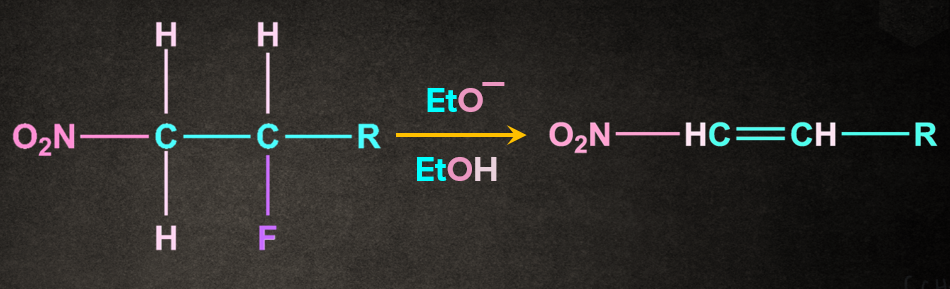
Conditions for E1cB Elimination
There are two conditions for any molecule to give E1cB elimination reaction:
1. A good electron withdrawing group must be present at the β-position to the leaving group.
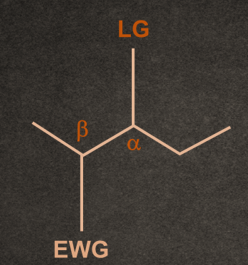
Example- Carbonyl (-C=O), nitro (-NO2), cyano (-CN), sulphonyl (-SO2-),
Phenyl (-Ph), ester (-COOR), and other carbonyl stabilizing groups.
2. Generally, the poor leaving group shows E1cB reaction.
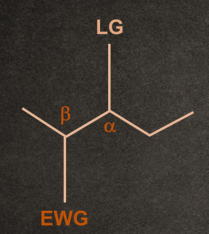
Example- ─F, ─OH, etc.
Mechanism of E1cB-Elimination
The mechanism of E1cB elimination reaction is a two-step process.
Step 1: Abstraction of proton to form carbanion
Base, EtO- abstracts the proton from the carbon to which electron withdrawing group, nitro(-NO2) is attached. The removal of the proton gives the carbanion intermediate.
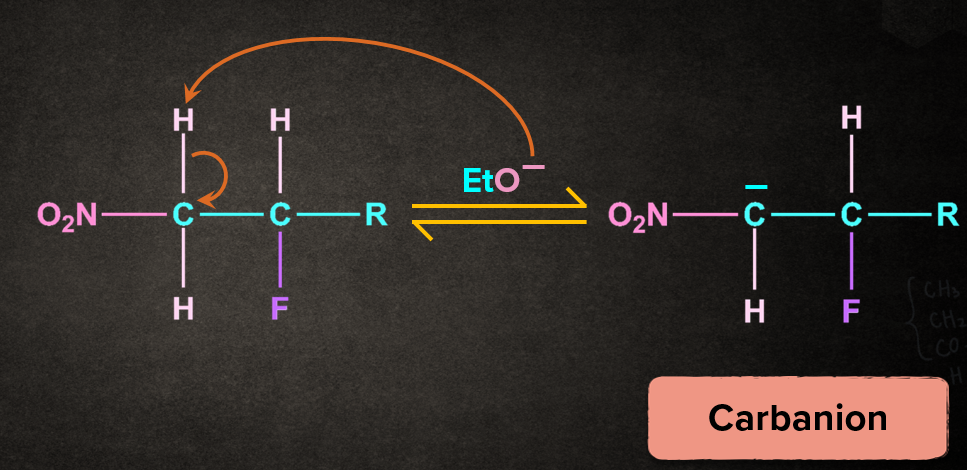
Step 2: Removal of leaving group to give an alkene
Leaving group, attached to the adjacent carbon of the carbon containing negative charge, leaves such that π-bond is formed between the two carbons containing the leaving group and negative charge.
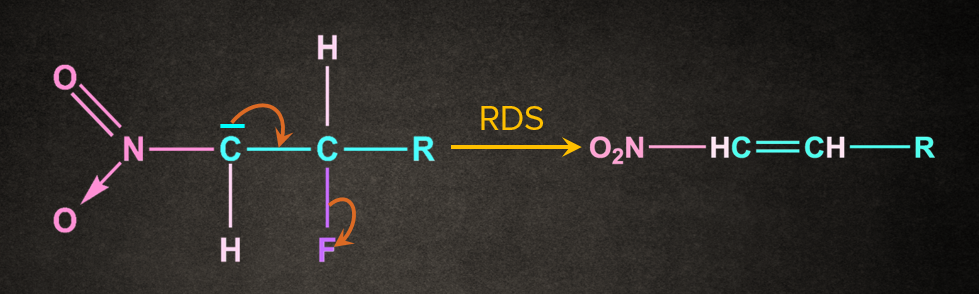
Rate of the Reaction in E1cB Mechanism
Second step, i.e. removal of the leaving group to form alkene, is a rate-determining step of the E1cB reaction. The reaction proceeds through the conjugate base of the starting material that’s why it is called as unimolecular elimination via conjugate base.
|
Rate of reaction ∝ [Conjugate base]1 |
The leaving group is not lost from the starting molecule, but from the conjugate base of the starting molecule. This sort of elimination, which starts with deprotonation, is called E1cB (cB for conjugate Base).
Practice Problems
1. More alkylated alkene is formed predominantly if the base is CH3CH2O-, while less alkylated alkene is obtained majorly when t-BuO- base is used. Which of the following statements is correct?
- t-BuO- is a bulky base, so the Hofmann product is formed as the major product.
- CH3CH2O- is a less bulky base, so the Saytzeff product is formed as the major product.
- t-BuO- is a bulky base, so the Saytzeff product is formed as the major product.
- Both A and B
Answer: D
Solution: t-BuO- is a bulkier base than CH3CH2O-. Bulky bases give product according to the Hofmann rule, which is the less substituted alkene. On the other hand, a more substituted alkene is obtained according to the Saytzeff rule if the base is less bulky.
So, option D) is the correct answer.
2. What are the factors that determine the stability of the product in an E1 elimination reaction?
- Stability of carbocation
- Stability of alkene formed
- Both A and B
- None of the above
Answer: C
Solution: The formation of the carbocation is the rate-determining step in the E1 elimination reaction. The more the stability of the carbocation intermediate, the higher the rate of the reaction. The increasing order of stabilities of carbocations is:
Primary carbocation < Secondary carbocation < Tertiary carbocation
The major product is decided with the help of the Saytzeff rule. According to this rule, “The most substituted alkene is the major product. Generally, it is the most preferred one.”
So, option C) is the correct answer.
3. According to which rule, the more substituted alkene will be formed as a major product?
- Markovnikov’s rule
- Saytzeff rule
- Anti markovnikov’s rule
- Hofmann rule
Answer: B
Solution: According to the Saytzeff rule, the most substituted alkene is the major product. Generally, it is the most preferred one.
So, option B) is the correct answer.
4. Which of the following is the rate-determining step in the E1cB mechanism?
A). Abstraction of proton to form carbanion
B). Removal of leaving the group to form alkene
C). Both A and B
D). None of the above
Answer: B
Solution: The mechanism of the E1cB reaction is a two-step process.
1. Abstraction of proton to form carbanion
2. Removal of leaving group to form alkene
Step two out of two steps is the rate-determining step.
Frequently asked Questions(FAQs)
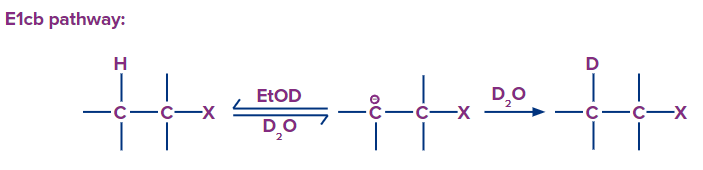
1. If EtOD is used as a solvent, then deuterium exchange takes place in the E1cB mechanism. Why?
Answer: In the E1cB mechanism, carbanion formed as an intermediate in the first step, which is reversible in nature. I.e, deuterium exchange takes place.
2. How does an electron withdrawing group stabilize the carbanion?
Answer: The electron withdrawing group stabilize the carbanion by -R/-M effect.
3. How to know whether the major product formed is according to the Hoffman or Saytzeff rule in - elimination?
Answer: In the case of E1 elimination, the reaction occurs in presence of a weak base like H2O, and a more substituted alkene will be formed as the major product according to the Saytzeff rule. On the other hand, in the case of E2 elimination, if the reaction occurs in the presence of a bulky base or poor leaving group, the least substituted product will be formed as the major product, according to the Hofmann rule.
4. Why is the least substituted alkene formed in the presence of a bulky base?
Answer: Bulky base abstracts the proton from the least hindered side. So, it abstracts the proton from that carbon which has a maximum number of hydrogen atoms, thus giving the least substituted alkene as the major product.


