-
Call Now
1800-102-2727
Baeyer’s Reagent–Definition, Preparation, Chemical Properties, Oxidation of Alkenes and Alkynes by Baeyer’s Reagent, Practice Problems & FAQs
What we perceive is what we rely upon. This conception is indeed so true that it has almost moulded our minds towards believing and acting majorly depending upon our visual perceptions. Graphics, changing colours, vibrant posters, seasonal transitions in nature— there are visual treats on almost every nook and corner of our universe. And this is a delight to watch!
Colours and conceptions have a great chemistry you see! To simplify this, I may say that colours have a great role to play in recognition and identification of various chemical compounds. It's magical how transitions in colours and changes in one colour to another is a significant marker of chemical reactions. Many such chemical compounds that are used for qualitative identification of certain compounds have a certain specific colour, which on reaction with a certain type of compound shows a distinct colour change, thereby making identification easy!

Baeyer’s reagent is one such important oxidising solution which is purple in colour. And suddenly goes colourless when it reacts with unsaturated hydrocarbons!
Let us understand this ‘magical reagent’ in detail.
Table of Contents
- What is Baeyer’s Reagent?
- Preparation of Baeyer’s Reagent
- Properties of Baeyer’s Reagent
- Reaction of Alkenes with Baeyer’s Reagent
- Reaction of Alkynes with Baeyer’s Reagent
- Procedure of Baeyer’s Reagent Test
- Application of Baeyer’s Reagent
- Practice Problems
- Frequently Asked Questions-FAQs
What is Baeyer’s Reagent?
In qualitative organic analysis, a cold, alkaline solution of KMnO4 is utilised to check for unsaturation. As a tribute to the German organic scientist Adolf von Baeyer, it is occasionally referred to as Baeyer's reagent.
Cold, alkaline potassium permanganate (KMnO4) in a violet-colored solution is the Baeyer's reagent. It has a powerful oxidising effect. The colour of this solution fades away and turns colourless when it interacts with a hydrocarbon having a double bond. Baeyer's reagent is used to test for the presence of alkenes and alkynes.
A powerful oxidising reagent called Baeyer's reagent is used to determine if a hydrocarbon has double or triple bonds. As a result, it is used for identification of unsaturated hydrocarbon molecules. For instance, if purple coloured KMnO4 is introduced to ethylene, it is oxidised to ethane-1,2-diol which is visualised as a colourless solution.
Preparation of Baeyer’s Reagent
First we shall see how potassium permanganate is produced.
Industrially, manganese dioxide, which also exists as the mineral pyrolusite, is used to make potassium permanganate. Production on a global scale was predicted to be 30,000 tonnes in 2000. In order to fuse the MnO2 with the potassium hydroxide, it must be heated either in air or with another oxygen source, such as potassium nitrate or potassium chlorate. Potassium manganate is produced by this technique.
The potassium manganate is then electrolytically oxidised in alkaline media to produce potassium permanganate:
Generally 1 % cold and alkaline KMnO4 solution is used as a Baeyer's reagent. Molar mass of KMnO4 is 158.034 g mol-1. So in order to prepare a 1 % cold and alkaline KMnO4 solution, the following steps are followed:
- To create a 1 % potassium permanganate solution, dissolve 1 g of solid KMnO4 in 100 mL of purified water.
- 10 g of anhydrous sodium carbonate (Na2CO3) is then added to the above KMnO4 solution, and the stoppered bottle is shaken until the Na2CO3 is completely dissolved and combined.
- Carbonate of sodium is a mild alkaline substance as it is a salt of weak acid (H2CO3) and strong base (NaOH). Solution will consequently become alkaline.
- When not in use, we need to store the solution in a cold and dark cabinet to maintain its freshness.
Properties of Baeyer’s Reagent
- Baeyer’s reagent is a cold, dilute and alkaline solution of potassium permanganate (KMnO4).
- It is a solution with a faint violet or purple hue.
- Purplish-pink tint of alkaline KMnO4 fades to a colourless solution with brown residues at the bottom, when double or triple bonds (-C=C- or -C≡C-) are made to react with it.
- While the permanganate () is reduced to manganese dioxide (MnO2), the alkene is oxidised to cis-1,2-diol.
- Because alkanes and aromatic compounds are unaffected by the action of Baeyer’s reagent and may therefore be distinguished from alkenes and alkynes, the reaction is noteworthy.
- Alkynes generally produces carboxylic acid on oxidation by Baeyer’s reagent along with brown residue to MnO2 and potassium permanganate (K2MnO4).
- Cold, dilute and alkaline KMnO4 is an effective oxidising reagent as Mn is in +7 (highest oxidation state) in this compound. So, it is used to evaluate if a hydrocarbon contains double or triple bonds. As a result, it can indicate whether the molecules of hydrocarbons are unsaturated or not.
- Oxidation state of Mn changes from +7 in permanganate () to +6 in manganate ions (). (electron transferred per mole= 1)


Reaction of Alkenes with Baeyer’s Reagent
Syn-1,2,-dihydroxylation of the double bond occurs when Baeyer’s reagent is reacted with alkenes. So the final product obtained is a cis-diol. For example- Ethene on oxidation using Baeyer’s reagent produces Ethan-1,2-diol. This is accompanied by formation of MnO2 and K2MnO4
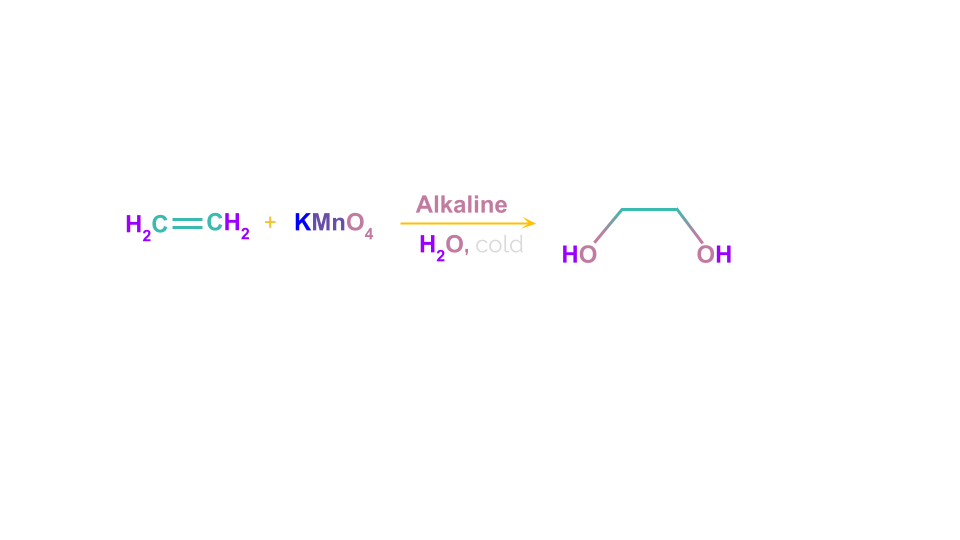
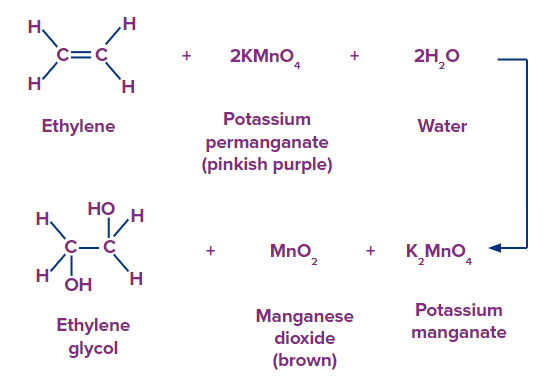
Mechanism:
A syn addition to the double bond results in the creation of a cyclic intermediate in this method. The freshly created -C-O bonds in the intermediates are then hydrolysed by water while maintaining their stereochemistry to produce cis-diols.
The first step in the process is the syn addition of a permanganate ion () across the same side of the alkene bond, resulting in the formation of an intermediate called a cyclic manganate ester. The cyclic ester is then hydrolyzed with water to produce a cis-diol with retained stereochemistry at the recently created C-O linkages.
The mechanism can be elaborated with an example of how cyclopentene is oxidised to cyclopent-1,2-diol as follows:

- Similar kinds of syn-dihydroxylation product is obtained by both Baeyer’s reagent and OsO4 in pyridine followed by treatment with NaHSO3.
- Mechanism for both are the same. Syn-stereochemistry is maintained in both and products are cis-diols.
- Comparatively speaking to osmium tetroxide (OsO4), potassium permanganate is less costly and safer. Also OsO4 is toxic to a certain extent.
- However, permanganate being a more potent oxidiser, there are chances for the diol to be over-oxidized as a result of its high oxidising properties, if the reaction conditions are not maintained well and the yields might be low then.
- So as a qualitative analytical approach, Baeyer’s reagent is better. But for production of cis-diol, we might prefer osmium tetraoxide.
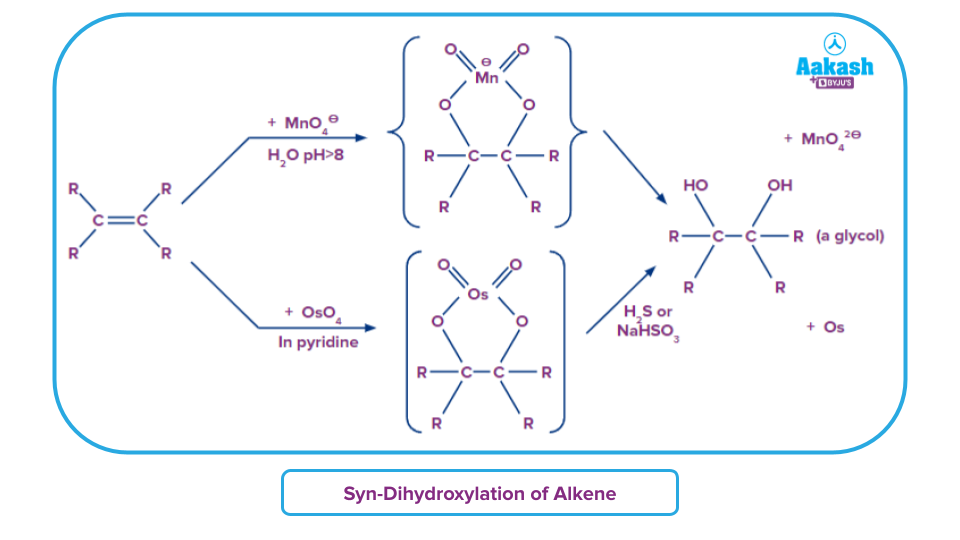
Alterations in product if reagent conditions for potassium permanganate are changed:
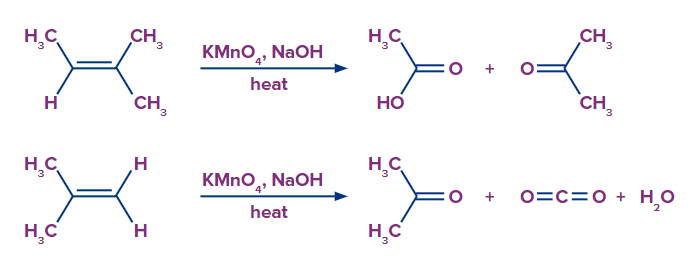
- Slight changes in reaction conditions can alter the pattern of products. For example, under acidic conditions, potassium permanganate produces different products. At this point the reagent is no longer termed as Baeyer’s reagent.
The alkene is cleaved into two products during strong oxidation with acidic potassium permanganate.
If unsaturated carbon is disubstituted, the product is a ketone.
If unsaturated carbon is monosubstituted, the product is a carboxylic acid.
If unsaturation is at the terminal, the product is carbon dioxide and water
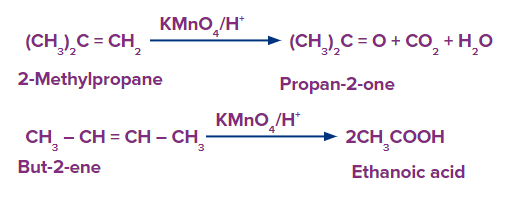
- When utilising KMnO4, there is one thing to be cautious about. Due to its potent oxidising properties, it may split the diol's C-C bond and further oxidise it into a carbonyl or carboxylic acids. It always accomplishes this in acidic and neutral solutions, hence using a basic permanganate solution at low temperatures is necessary for obtaining cis-diols.
- Hot solution will oxidatively cleave carbon-carbon double bond thereby generating ketones or acids depending on substituents. As a result, monosubstituted and disubstituted alkenes yield carboxylic acids and ketones, respectively, whereas terminal alkenes are oxidised to produce carbon dioxide.
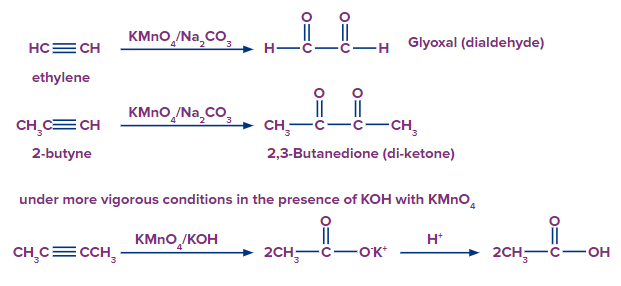
Reaction of Alkynes with Baeyer’s Reagent
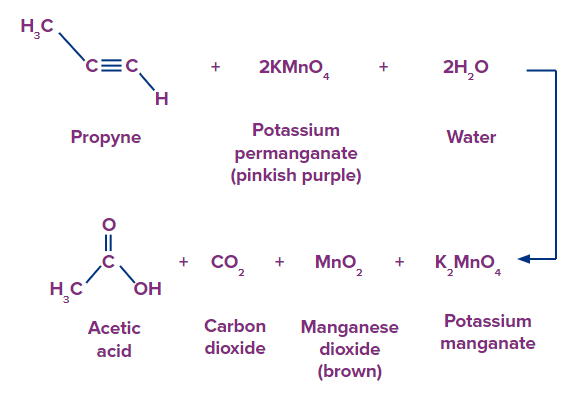
Alkyne compounds can be oxidised by Baeyer's reagent into vicinal diketones or dialdehydes (Vic-diketones or 1,2-diketones), or under more strongly alkaline solutions, to carboxylic acids.
In order to create more vigorous conditions, the solution's alkalinity can be increased by adding potassium hydroxide (KOH) which is an effective alkali.
Procedure of Baeyer’s Reagent Test
- In a tiny test tube, combine 4 drops(40 mg) of the sample with 1 mL of ethanol (or 1,2-dimethoxyethane).
- Put on gloves and add three drops of the 1 % deep purple coloured cold, dilute and alkaline solution of KMnO4 (as prepared freshly-mentioned above) to the test tube.
- There is a high probability of staining and excessively drying out the skin of your hands (this reagent is caustic and will colour skin brown!). So be cautious.
- The test tube should be agitated, then let it sit for one minute. The emergence of a brown precipitate or hue along with decolourising the purple solution to give a colourless solution, is a positive result which means the sample contains unsaturation.
- Dark purple colour with no precipitate (unreacted KMnO4) indicates a negative test which means absence of alkene or alkyne.
Application of Baeyer’s Reagent
- Baeyer’s reagent is majorly used as an analytical reagent to distinguish unsaturated hydrocarbons from alkanes and other aromatic compounds. This is used to distinguish between saturated (alkane) and unsaturated (alkene/alkyne) hydrocarbons.
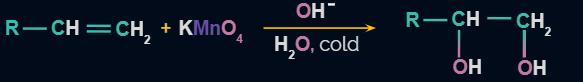
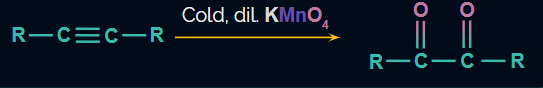
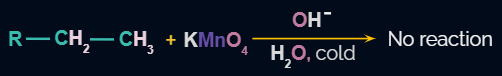
In the first two reactions purple colour disappears and in the third reaction, the purple colour of the cold and dilute KMnO4 solution is retained.
- Potassium permanganate has several categories of utilities such as applications in medicine, treatment of water, organic compound synthesis, preservation of fruit etc.
- A quantitative measurement of the total oxidisable organic material in an aqueous sample may be made using potassium permanganate.
- When conducting redox titrations in analytical chemistry, a standardised aqueous solution of KMnO4 is occasionally employed as an oxidising titrant (permanganometry). The solution turns a faint shade of purple when the potassium permanganate is titrated, and as more excess titrant is added, the colour of the solution darkens.
- Oxalic acid reduction is frequently used to standardise KMnO4 solutions. It is employed in agricultural chemistry to estimate the amount of soil active carbon.
- Even at high temperatures, ethylene absorbents help bananas last longer in storage (helps in delayed ripening). Bananas with potassium permanganate can be packed in polyethylene to take advantage of this effect. The permanganate prolongs the fruit's shelf life by eliminating ethylene through oxidation, extending it by up to 4 weeks without refrigeration.
Recommended Videos
https://www.youtube.com/watch?v=MRHfT0LANe8
Practice Problems
Q.1 Which other reagent apart from Baeyer’s reagent can bring 1,2, dihydroxylation of alkene?
Answer: (A)
Solution: Syn-1,2-dihydroxylation product is obtained by both Baeyer’s reagent (Cold, dilute and alkaline KMnO4) and OsO4 in pyridine followed by treatment with NaHSO3. Acidic potassium permanganate oxidises alkenes to produce multiple varieties of products like ketones, aldehydes or carboxylic acids depending on the substituents. So Option A is the correct answer.
Q.2 What is the drawback of Baeyer’s reagent?
Answer: The tendency of KMnO4 being a strong oxidiser is to overoxidized the alkene and cause cleavage at the C=C double bond is very high. Therefore it may react with a variety of functional groups. So, bromine water is more suited for quantitatively assaying unsaturation (double or triple bonds) present in a hydrocarbon.
Example: aldehydes also get oxidised by this reagent to give carboxylic acid.
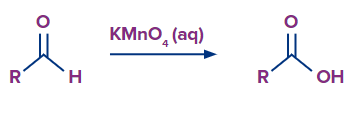
Q.3 What is oxidised and what is reduced in Baeyer’s reagent?
Answer: Unsaturation (alkenes and alkynes) or oxidisable functional groups (aldehydes and some alcohols) are oxidised by Baeyer’s reagent. Permanganate ion on the other hand is reduced from +7 state in to +6 state in .
The brown precipitate (MnO2) that results from reduction of the deep purple permanganate ion ( ). Alkenes and aromatics cannot react with permanganate, making it a useful test for differentiating between the two.
Frequently Asked Questions-FAQs
Q.1. Why is a mild alkaline solution of Na2CO3 used and not a strong alkaline solution of KOH in KMnO4?
Answer: Under strongly alkaline conditions chances of over-oxidation of C=C are quite high.
As permanganate (Mn7+) is only reduced to manganate (Mn6+), oxidation in strongly alkaline media is least effective in terms of transferred electrons per mole. So during this oxidation process, MnO4- can take up only one electron per mole and hence becomes a weaker oxidising agent. Under milder conditions the oxidising ability remains uncompromised also product of alkene oxidation sticks to cis-diols only at milder alkaline.
Q.2. What is the effect of Baeyer’s reagent on skin?
Answer: Potassium permanganate is a potent oxidiser. If skin contact occurs then there is a long lasting brown stain. So it is excessively drying on skin and also stains skin, brown. So we should take proper precautions while working with it.
When potassium permanganate is applied to the skin in mild forms, it typically kills germs by releasing oxygen when it comes into contact with compounds in the skin. Additionally, it functions as an astringent, a drying agent.
Q.3. Is Baeyer’s test and Bromine water test same?
Answer: No they aren’t the same. The bromine water test is a qualitative method for determining whether the compound contains alkene or alkane functional groups.
Alkene groups undergo an addition reaction with bromine water () in the dark, resulting in a decolorised solution.
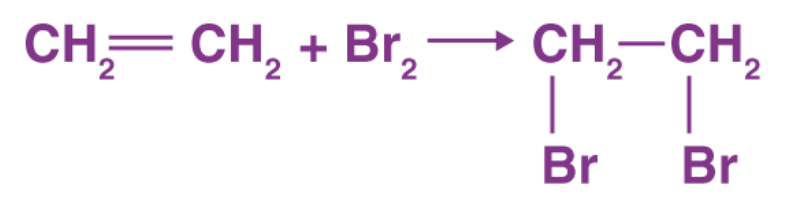
Alkane, however, shows no reaction with bromine water and leaves the colour of the water alone. The most typical substances that are subjected to a bromine water test are enols, alkenes, aniline, glucose, phenols, and acetyl groups.
Q.4. What happens when Baeyer’s reagent is evaporated?
Answer: Upon slow evaporation, it leaves behind prismatic, glittering, purplish-black coloured crystals of potassium permanganate.
Q.5 Why does the metal centre of ions interact with the double bond present in an unsaturated compound?
Answer: The oxidation state of manganese in is +7. Hence Mn7+ which is its highest oxidation state, is a highly electrophilic metal centre. Therefore an electrophilic metal atom's vacant d-orbitalextends far beyond the surrounding O-atoms and results in an electron transfer from the double bond of the hydrocarbon to the metal centre. This interaction is completed by back-bonding of the nucleophilic oxygens to the antibonding *-orbital of the unsaturated hydrocarbons.


