-
Call Now
1800-102-2727
Average Atomic Mass-Average Molecular Mass: Definitions, Examples, Practice problems & FAQs
If you have an equal volume of normal breathable atmospheric air and moist air, which of the following weigh high and why?
In direct comparison, we start thinking moist air must be heavier than dry air but if you think our normal air consists of 78% nitrogen gas and 21% oxygen gas. Moist air must contain vapours of water, you know the molar mass of water is 18 g mol-1 which is lighter than both major components of air nitrogen gas (28 g mol-1) and oxygen gas (32 g mol-1). In moist air water vapours must replace these gas for equal volume, so moist air is lighter than normal air.
Table of contents
- Average atomic mass
- Isotopes
- Percentage abundance
- Average molecular mass
- Practice problems
- Frequently asked questions-FAQs
Average atomic mass:
In the case of elements, a sample consists of more than one kind of atom called isotopes. Therefore, the Mass of a sample of atoms is also represented as weighted average mass and is called average atomic mass.
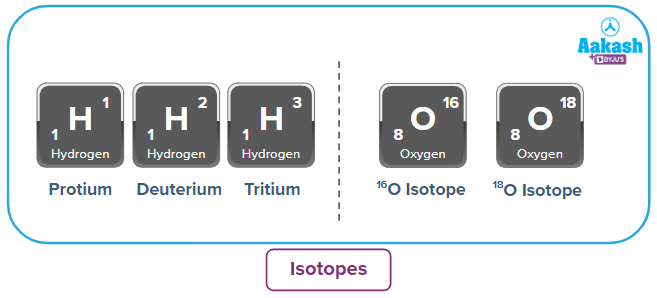
Isotopes:
Isotopes are those particles that have the same number of protons but different numbers of neutrons.
Percentage abundance:
Percentage abundance is defined as the percentage value of the number of isotopes available in nature for a given element.
So, the weighted average atomic mass of an element is determined by multiplying the relative abundances of the element's isotopes by their atomic masses and then the summation of products.
average atomic mass =(% abundance)1 Mass1 + (% abundance)1 Mass2 +………………
Average atomic mass
Example 1.
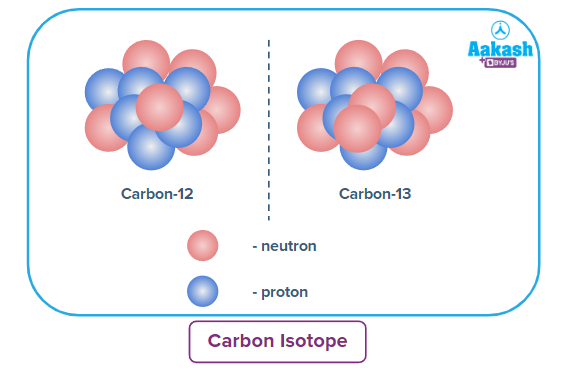
Abundance of Carbon-12 isotope → 99%
Abundance of Carbon-13 isotope → 1%
Average atomic mass of carbon = n=1n[(99) (12)] + [(1) (13)]100=12.01 amu
Average molecular mass
When a set of compounds is given along with the number of molecules of each compound, The average molecular mass can be calculated as follows.
Average molecular mass =
the Average molecular mass =
Practice problems
Q 1. What is the average atomic mass of chlorine if it contains two types of atoms having masses of 35 amu and 37 amu. The relative abundance of these isotopes in nature is in the ratio of 3:1.
a. 35.0
b. 37.0
c. 36.0
d. 35.5
Answer: (D)
Given, ratio if Cl35 & Cl37is 3:1
So, % of Cl35 =33 + 1100=75%
% of Cl37 =13 + 1100=25%
Average atomic mass =
Average atomic mass of chlorine =
Q 2. Given that the abundances of isotopes Fe54, Fe56 and Fe57 are 5%, 90%, and 5% respectively, 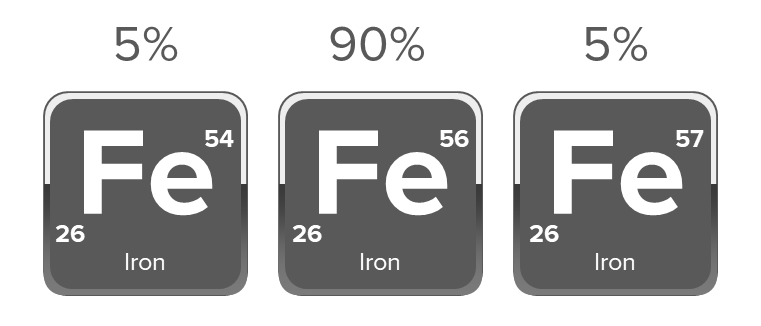
calculate the average atomic mass of Fe
a. 54.78
b. 56.65
c. 55.95
d. 55.67
Answer: (C)
Given, abundances of isotopes, Fe54, Fe56 and Fe57 are 5%, 90% and 5%
Average atomic mass =
Average atomic mass of Fe =
Q 3. What is the average mass of air if it contains 80 % nitrogen gas by volume and 20 % oxygen gas by volume?
a. 28.8 g mol-1
b. 32.0 g mol-1
c. 28.0 g mol-1
d. None of these
Answer: (A)
So, % of N2 gas = 80%
% of O2 gas = 20%
We can conserve mass;
Mass of the mixture (air) = mass of N2 gas + mass of O2 gas
We know, d=mV and PM = dRT, d=PMRT
a
At a constant Temperature (T) and Pressure (P)
Q 4. If a gas mixture contains 560 g of N2 gas and 320 g of O2 gas, find the average molecular mass of the mixture.
a. 32.00 amu
b. 29.33 amu
c. 28.00 amu
d. 28.8 amu
Answer: (C)
Mole of N2 gas =
Mole of O2 gas =
Average molecular mass =
Average molecular mass =
Average molecular mass of mixture =
Frequently asked questions-FAQs
Q. What is the unit of average atomic mass and average molecular mass?
Answer: Average atomic mass and average molecular mass is expressed in ‘u’ or ‘amu’.
Q. How can we calculate the average molecular mass?
Answer: The number average molecular weight is the total weight of the sample divided by the number of molecules in the sample. The Average Molecular Weight of a mixture is computed from the molar composition and the molecular weight.
Q. If relative atomic mass can not be a fraction why somewhere is fractional atomic mass or molar mass mentioned, e.g- Cl35.5
Answer: Cl35.5 written because of its isotopes, Cl present in nature in different isotopes in different natural abundance (%). So, due to their isotopes, they have fractional mass. We can call it weighted average atomic mass.
Q. What is the difference between atomic weight and atomic mass?
Answer: Atomic mass is generally used for single isotopes but atomic weight is generally the weighted average of isotopic abundance in nature. Atomic mass is always a whole number because it has a relation with the number of protons and neutrons in the nucleus.
Q. What is the ideal gas equation?
Answer: the ideal gas equation is PV=nRT
Where, P=pressure of gas
V= volume, n= moles, R=universal gas constant & T = Temperature in K
We know, number of moles = and


