-
Call Now
1800-102-2727
Atomic Radii & Ionic Radii of p-Block Elements - Factors Affecting Atomic Radii, Trends in Atomic Radii, Practice Problems and FAQ
Your class might consist of many pupils, isn't it? And you must have observed a lot of variation among your classmates, for sure! Be it in terms of facial characteristics, measurements, shapes and sizes or even their hairstyles!
Try to recall, have you ever seen your class being grouped in a continuous trend of certain physical characteristics?
Yes! If not anywhere else, you and your peers must have been made to stand in ascending order of heights, in the auditoriums, or playgrounds, during morning assemblies. Probably you do that every day at your school.

So is the case with the 118 elements in the modern periodic table who also follow a pattern in terms of atomic size (with certain exceptions of course!). Just like the multiple classes that constitute a school, each block of elements constituting the periodic table, i.e., s-block, p-block, d-block and f-block has a certain trend and its own exceptions regarding the atomic sizes of their elements. We shall now be trying to understand and decode more about the atomic and ionic sizes of elements of the p-block in particular on this page.
Let’s begin!
TABLE OF CONTENTS
- Atomic & Ionic Radii of p-Block Elements
- Factors Affecting Atomic Radii
- Group 13 - Atomic Radii & Ionic Radii
- Group 14 - Atomic Radii & Ionic Radii
- Group 15 - Atomic Radii & Ionic Radii
- Group 16 - Atomic Radii & Ionic Radii
- Group 17 - Atomic Radii & Ionic Radii
- Group 18 - Atomic Radii & Ionic Radii
- Practice Problems
- Frequently Asked Questions - FAQ
Atomic & Ionic Radii of p-Block Elements
Group 13 to 18 of the periodic table constitute the p–block. p–block contains metals, metalloids, as well as, non–metals. The first member of each group from 13 to 17 of the p–block elements differ in many respects from the other members of their respective groups because of their small size, high electronegativity and absence of d-orbitals.

The atomic radius of p-block elements often decreases as one moves across a period in the periodic table from left to right. It is a result of the electron addition occurring inside the same valence shell and being more strongly pulled by the nuclear charge at each stage.
This can be shown as follows.
|
Element |
Boron |
Carbon |
Nitrogen |
Oxygen |
Fluorine |
|
Outer electronic configuration |
2s2 2p1 |
2s2 2p2 |
2s2 2p3 |
2s2 2p4 |
2s2 2p5 |
|
Nuclear charge |
+5 |
+6 |
+7 |
+8 |
+9 |
|
Effective nuclear charge |
+ 2.60 |
+ 3.25 |
+ 3.90 |
+4.55 |
+ 5.20 |
|
Atomic radius (pm) |
88 |
77 |
70 |
66 |
64 |
The atomic radius of the elements grows as the atomic number increases while travelling down a group. This is because when we travel from one element to the next down the group, the number of shells increases. The extra shell more than makes up for the increased nuclear charge.
Here are facts on various types of atomic radii.
- Metallic radii correspond to half the internuclear distance separating the metal cores in the metallic crystal having metallic nature. In p-block, it is applicable to metals like aluminium alone.
- Covalent radius is one-half of the distance between the centres of two nuclei (of like atoms) bonded by a single covalent bond. Covalent radius is generally used for non-metals. Unit: Picometre (pm) or Angstrom (Å)
- For noble gases, it is half the distance between the nuclei of two non-bonded nearest neighbouring atoms of the same element in its solid state.
- For non-metals, van der Waals radii is half the distance between the nuclei of two non-bonded nearest neighbouring atoms of two adjacent molecules of the same element in a solid state.
- van der Waals radius does not apply to metals. Its magnitude depends upon the packing of the atom when the element is in the solid state.
Factors Affecting Atomic Radii
- Number of shells
Atomic radii is directly proportional to the number of shells.
- Effective Nuclear Charge
Due to an increase in the attractive force acting on the outermost electrons, the relationship between atomic radius and effective nuclear charge is inverse.
- Screening Effect
In multi-electron atoms, the screening of the outermost electrons from the nucleus by the inner electrons is called the shielding or screening effect.
The order of shielding is s>p>d>f.
As a result, the nucleus's entire impact and attraction are not felt by the outermost electrons. Effective nuclear charge is the name given to the real charge experienced by electrons. As a result, the atomic radius grows as the shielding effect grows.
- Number of Bonds
The covalent radius depends on the number of bonds. With the increase in covalent bonds, atomic radius decreases. Hence, atomic radius is inversely proportional to the number of bonds.
Group 13 - Atomic Radii & Ionic Radii
Atomic radii as well as ionic radii of Group 13 increase from top to bottom with the exception that the atomic radii of Ga is less than that of Al.
The tendency of Group 13 elements to behave as Lewis acids decrease on moving down the group due to increase in atomic radius (decrease in effective nuclear charge) down the group, which makes them reluctant to accept additional electron pairs.

Because Group 13 elements have a higher nuclear charge than Group 2 elements, their atomic and ionic radii are lower than those of Group 2's alkaline earth metals. As you descend the group, gallium’s atomic radius becomes somewhat less than that of aluminium. This is because Ga contains d-electrons, which ineffectively shields the nucleus (poor shielding of d-orbitals). Because of this, the nucleus in Ga exerts a stronger force of attraction on the electrons than it does in Al, resulting in a somewhat smaller atomic radius for Ga (135 pm) compared to Al (143 pm).

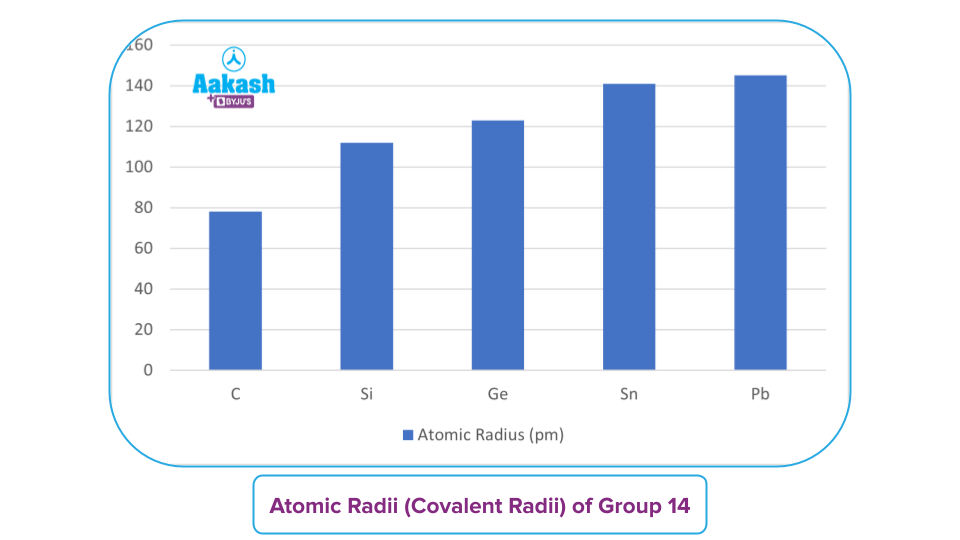
Group 14 - Atomic Radii & Ionic Radii
All group 14 members are solids. Carbon is a non-metal. Si & Ge are metalloids. Sn and Pb are metals. Covalent radius increases down the group.

There is a Considerable increase in covalent radius from C to Si, followed by a small increase from Si to Pb. This is due to the presence of completely filled d and f-orbitals in heavier members. Group 14 elements have smaller radii than Group 13 elements. The rise in the effective nuclear charge can account for this. The rise in radii is significant from C to Si, and thereafter it becomes less significant.
Group 15 - Atomic Radii & Ionic Radii
In group 15, Nitrogen (N) and Phosphorus (P) are non-metals, Arsenic (As) and Antimony (Sb) are metalloids and Bismuth (Bi) is a metal. Down the group, covalent and ionic radii in a particular state, increases. From N to P, There is a considerable increase in covalent radii. From As to Bi, only a small increase in covalent radius due to the presence of completely filled d and f-orbital in heavier elements.

Group 15 elements have lower atomic and ionic radii than their comparable group 14 elements. Atomic radii rise as atomic number increases as you move down the group. The nuclear charge of elements in group 15 is higher than that of elements in group 14, as explained. Atomic radii shrink as a result of the electrons' strong attraction to the nucleus caused by a rise in nuclear charge. As a result, group 15 elements have smaller atomic radii than Group 14 elements. As you move along the group, the number of shells increases because each subsequent element gains a new primary shell. However, only a little increase in covalent radius is seen from As to Bi. This is because these heavier elements include fully filled d and/or f-orbitals.
Group 16 - Atomic Radii & Ionic Radii
Group-16 elements have lower atomic radii than equivalent group-15 elements. The rise in effective nuclear charge is to blame for this. Moving down the group in group-16, causes the atomic and ionic radii to rise as additional electron shells are added at each succeeding element. As we go from oxygen to polonium, the atomic and ionic radius increases. The chalcogen with the smallest atomic and ionic radius (apart from livermorium) is oxygen, whereas the chalcogen with the biggest atomic and ionic radius is polonium.

Additionally, it should be noted that the addition of protons and a rise in the effective nuclear charge cause an overall reduction in the atomic radius of elements over time. Therefore, compared to lithium, oxygen will have an atomic radius that is substantially lower.
Group 17 - Atomic Radii & Ionic Radii
In each of their respective eras, the members of group 17 have the shortest atomic radii. They are said to have a maximum effective nuclear charge, which explains why. As a result of an increase in the number of quantum shells, atomic and ionic radii in a group rise from fluorine to iodine.
Atomic radii are a unit of measurement for the separation between an atom's nucleus and its outermost electron-containing shell. Ionic radii serve as a gauge for anionic size (X-). The atomic and ionic radii tend to grow as we move down the group when an additional energy shell is introduced. The explanation for why the atoms in this group have lower atomic radii than other elements is high atomic charge

Ionic and atomic radii are both calculated using the separation between the nucleus and the outermost electron. For non-metals, we majorly consider covalent radii when we talk of atomic radii. Halogens have the lowest radii among the other elements in their respective rows since they are members of Group 17 and contain seven electrons in their outermost orbital. This is due to the fact that the more the number of electrons, the more will be the nucleus's attraction.
It is crucial to remember that when the number of atomic shells rises, the atomic radii of the elements lower in the group continue to grow. Ionic radii correspond to the halide ions (e.g. F-, Cl-, Br-, I-).
|
Element |
Covalent Radii (in pm) |
Ionic Radii (in pm) |
|
F |
64 |
133 |
|
Cl |
99 |
184 |
|
Br |
114 |
196 |
|
I |
133 |
220 |
Group 18 - Atomic Radii & Ionic Radii
The atomic radii of group 18 members are quite tiny. Due to the addition of additional shells, the atomic radii of noble gases grow down the group as the atomic number rises.
The melting temperature, boiling point, enthalpy of vaporisation, and solubility all increase as one moves down Group 18 due to an increase in atomic radius and interatomic forces.

The electron clouds of these non-polar atoms get more and more polarised as the atoms' atomic sizes increase along the group, which results in weak van der Waals interactions between the atoms. Because of their lower melting and boiling temperatures, these heavier elements may thus form liquids and solids more readily.
The atomic radius of inert gases is the largest in its period. Their radius is represented by the van der Waals radius (as they exist in a monatomic state). Generally, this is larger than the covalent and metallic radius. Down the group atomic radii increase.
Practice Problems
1. Among phosphorus, antimony, and bismuth, which will have the largest atomic radius?
a. Phosphorus
b. Antimony
c. Bismuth
d. All are equal
Answer: C)
Solution: Atomic size increases on going down the group due to the addition of shells. Hence, bismuth will have the largest size among the given elements.
So, option C) is the correct answer.
2. Which element has the smallest atomic radius?
a. Aluminium
b. Gallium
c. Thallium
d. Boron
Answer: D)
Solution: The size of atoms increases down the group. So, out of these elements of Group 13, boron will have the smallest size as it is the first element in its group.
So, option D) is the correct answer.
3. Which group has a greater atomic size than halogens?
a. Noble gas
b. Chalcogens
c. Pnictogens
d. Transition metals
Answer: A)
Solution: The size of group 18 elements is observed to be the largest in the period because the size is determined in terms of van der Waals radius (exists in a monatomic state) that is generally larger than the covalent (or metallic) radius. In fact, the radii of noble gases should be compared not with the covalent radii but with the van der Waals radii of the other elements. Inert gases are monatomic. So, the covalent radii and metallic radii will not be applicable for them. Therefore, for inert gases, van der Waals radius is used to determine size.
So, option D) is the correct answer.
4. Why is the ionic radii of Ga3+ lower than Al3+ ?
Solution: Gallium contains inner d - electrons which do not shield the nuclear charge effectively. Consequently, the outer electrons in gallium experience a greater force of attraction by the nucleus than in aluminium. Hence, the ionic radius of Ga3+ is slightly less than that of Al3+
Frequently Asked Questions - FAQ
1. Why does Ne have a larger atomic radius than F?
Answer: As we transition from halogen (F) to the inert gas (Ne), the atomic radius abruptly rises. Due to the complete filling of all orbitals in inert gases, which causes the electronic repulsions to be at their maximum, as well as the weaker van der Waals interaction in the mono-atomic gaseous atoms of the 18th group of elements, this is the case.
2. Which of the following orbitals, namely s,p,d, and f has the highest screening power?
Answer: Since they are the ones closest to the nucleus, s-orbitals have the maximum screening power. Consequently, the nucleus strongly attracts the electrons, making it difficult to free electrons with s-orbital energy. Although the p-orbitals have a strong shielding power as well, it is smaller than other orbitals in comparison. In contrast, the shielding power in d-orbitals is lower since it is farther away from the nucleus. In contrast to all the other orbitals, the f-orbitals have the least amount of shielding power. They are the farthest from the nucleus and have the least attraction to it.
3. What is the relation between atomic radius and covalent radius?
Answer: Covalent radii is a type of atomic radii. A single atom has an atomic radius. When talking about two such adjacent atoms’ internuclear distance, we call atomic radii. It is the distance between the nucleus's centre and the edge of its electron cloud. When two atoms of the same species are joined, covalent radius is applicable. It is halfway between the two nuclei away. So they are the same in a way.
4. What is the relation between covalent radius and van der Waals’ radii?
Answer: A portion of the electron cloud becomes common because a covalent connection is created by the overlapping of two half-filled atomic orbitals. van der Waals radii is half the distance between the nuclei of two non-bonded nearest neighbouring atoms of two adjacent molecules of the same element in a solid state. Covalent radii are, therefore, always lower than van der Waal radii.


