-
Call Now
1800-102-2727
Electrophilic Aromatic Substitution - Introduction, Types and Mechanism of Electrophilic Aromatic Substitution, Practice Problems & FAQs
Have you ever traveled in a metro or in a bus or in a local train?
Getting a seat in a metro always feels like a victory. Sometimes when you see that the seats which are already reserved for senior citizens are vacant , then we usually sit on it. But suppose at the very next metro station if any senior citizen/differently abled person will come then he/she has a complete authority to replace you.

Similarly by considering this as an analogy for electrophilic aromatic substitution reaction, where an electrophile replaces an atom attached to an aromatic ring .
Let's study in detail what are electrophilic aromatic substitution reactions and what all types of electrophilic aromatic substitution reactions are possible!
TABLE OF CONTENT
- Electrophilic Aromatic Substitution Reaction
- Mechanism of Electrophilic Aromatic Substitution Reaction
- Types of Electrophilic Aromatic Substitution Reaction
- Practice Problems
- Frequently Asked Questions
Electrophilic Aromatic Substitution Reaction:
Organic reactions in which an electrophile replaces an atom attached to an aromatic ring are known as electrophilic aromatic substitution reactions. These ractions typically involve the replacement of a hydrogen atom from a benzene ring with an electrophile.
An electrophilic aromatic substitution reaction preserves the aromaticity of the aromatic system. For example, when bromobenzene is formed from the reaction of benzene and bromine, the aromatic ring's stability is not lost. This reaction is depicted in the diagram below.
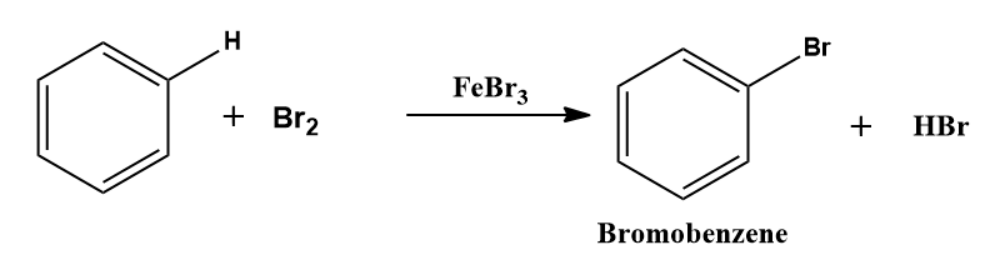
Because benzene is an electron-rich system with delocalized-electrons, it undergoes electrophilic substitution reactions.
The general reaction of electrophilic aromatic substitution can be represented as:
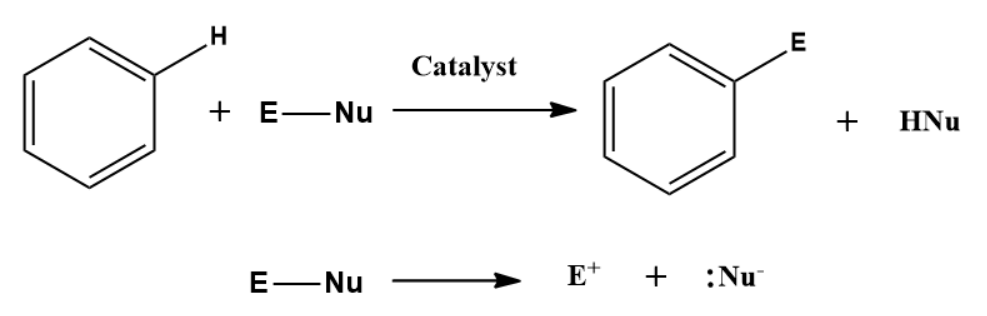
Mechanism of Electrophilic Aromatic Substitution Reaction:
The mechanism of electrophilic aromatic substitution reaction majorly contains three steps, which are as follows:
Step 1: Generation of an Electrophile
In the generation of an electrophile, a lewis acid is used. Anhydrous aluminium chloride is a very useful Lewis acid in the generation of electrophiles from aromatic ring in the halogenation, alkylation, and acylation. As shown below, the resulting electrophiles (from the combination of anhydrous aluminium chloride and the attacking reagent) are Cl+, Br+, R+, R-CO+.
Following are the electrophiles used in the electrophilic aromatic substitution reaction:
|
Electrophile (E+) |
Name |
Source |
Name of reaction |
|
Cl+ |
Chloronium |
Cl2+AlCl3 |
Chlorination |
|
Br+ |
Bromonium |
Br2+AlBr3 |
Bromination |
|
NO2+ |
Nitronium |
HNO3+H2SO4 |
Nitration |
|
SO3 |
Sulfur trioxide |
Fuming H2SO4 |
Sulphonation |
|
R+ |
Alkyl Carbocation |
RX+AlX3 X = Cl, Br |
Friedel-Crafts Alkylation |
|
R-CO+ |
Acyl Carbocation |
RCOCl+AlCl3 |
Friedel-Crafts Acylation |
Step 2: Formation of Arenium ion(Carbocation)
In the second step, the electrophile formed in the first step will attack on the benzene ring to form an intermediate carbocation which is called as -complex or arenium ion.
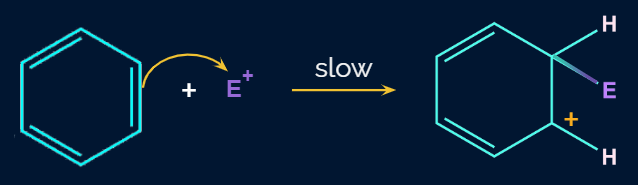
The formed arenium ion is resonance stabilized. Because electron delocalization terminates at the sp3 hybridized carbon, the sigma complex or arenium ion loses its aromatic character. The resonance forms of arenium ions are:
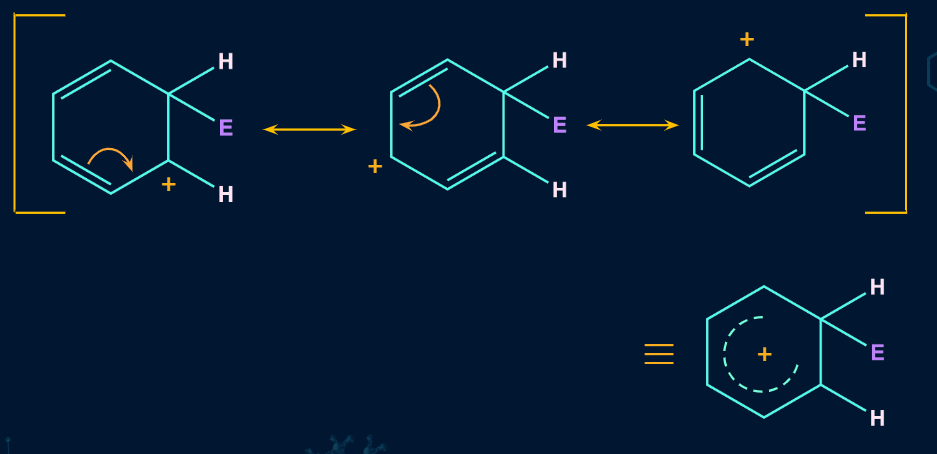
This step is a slow and rate-determining step and the rate of electrophilic aromatic substitution reaction depends on the slow step.
As two molecules are involved in the rate-determining step, so electrophilic aromatic substitution reaction is bimolecular.
Step 3: Removal of Proton
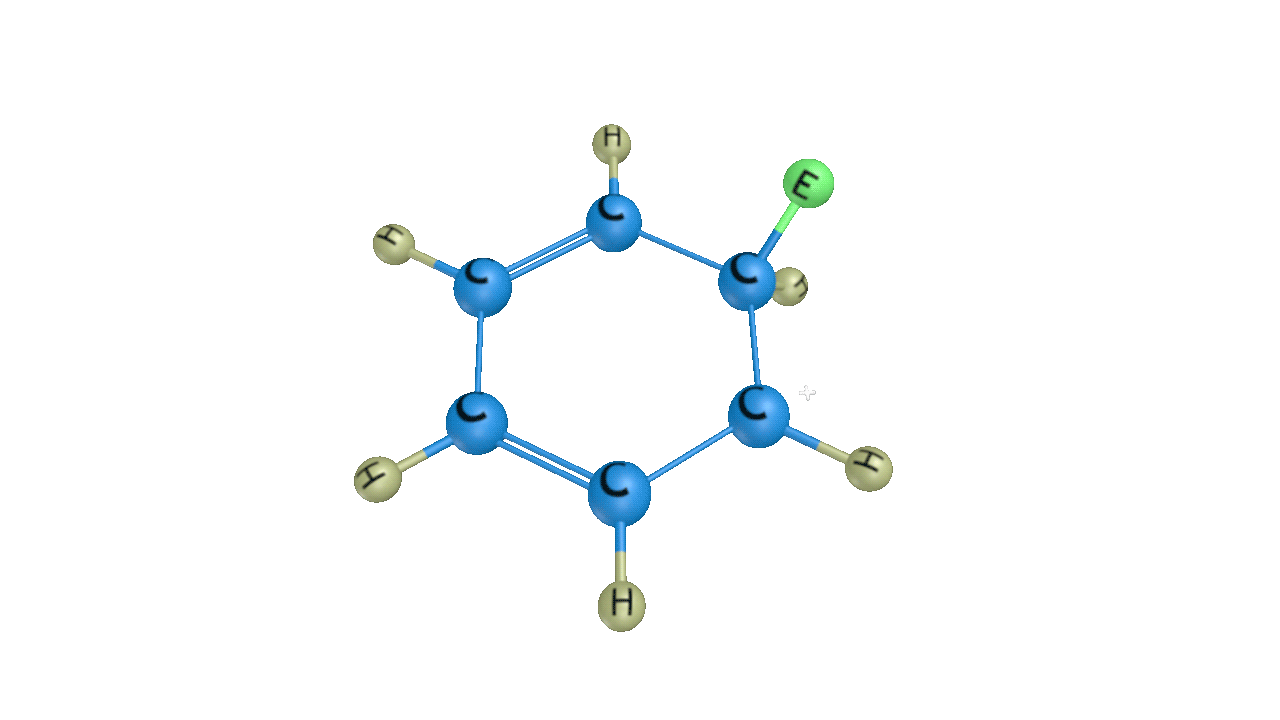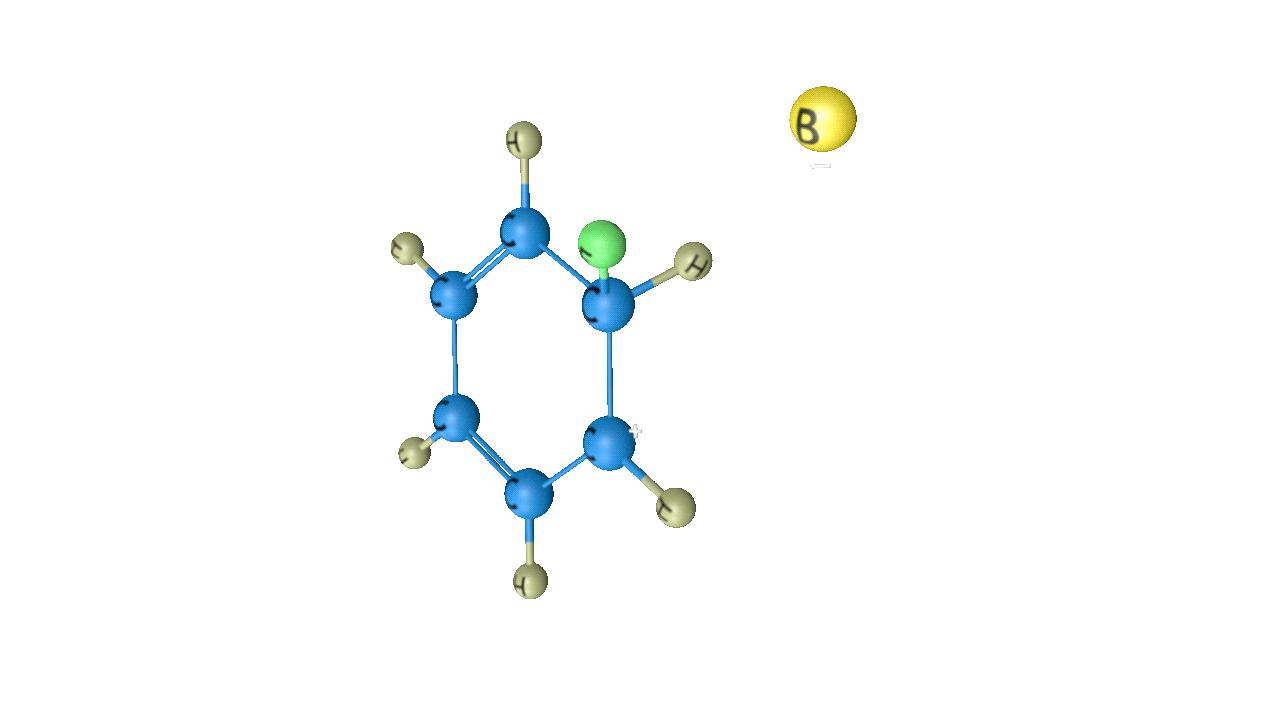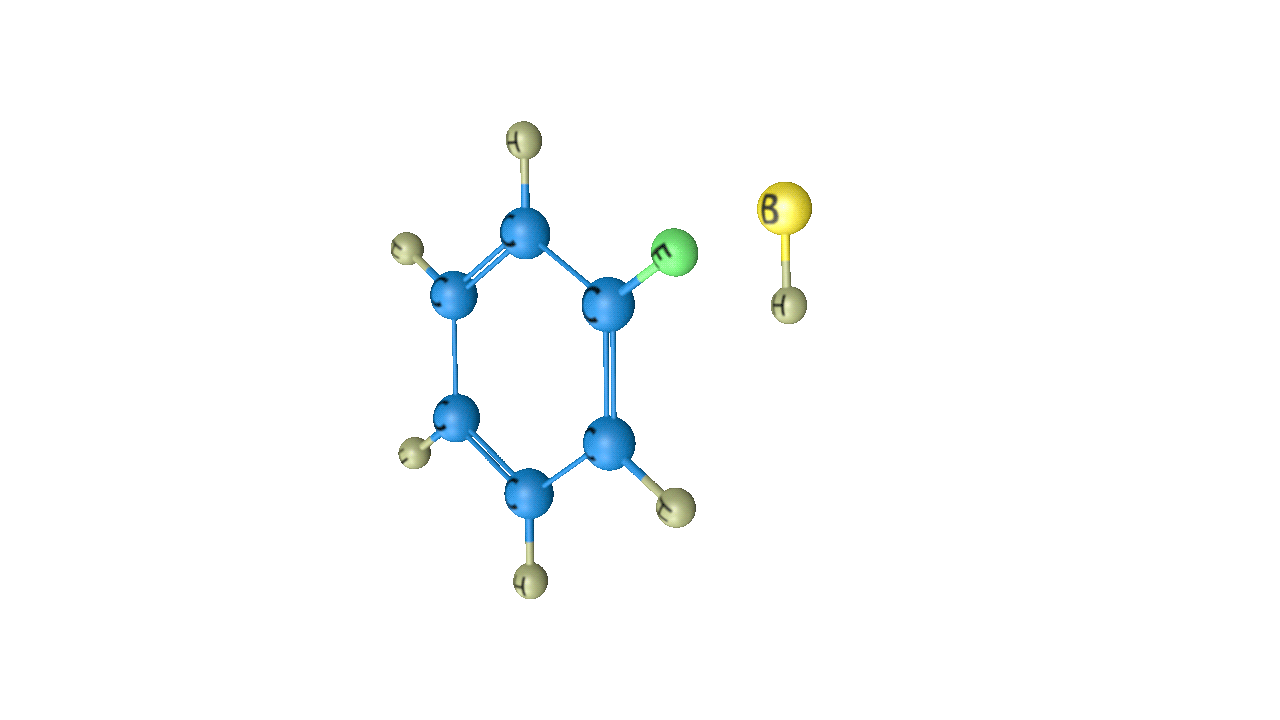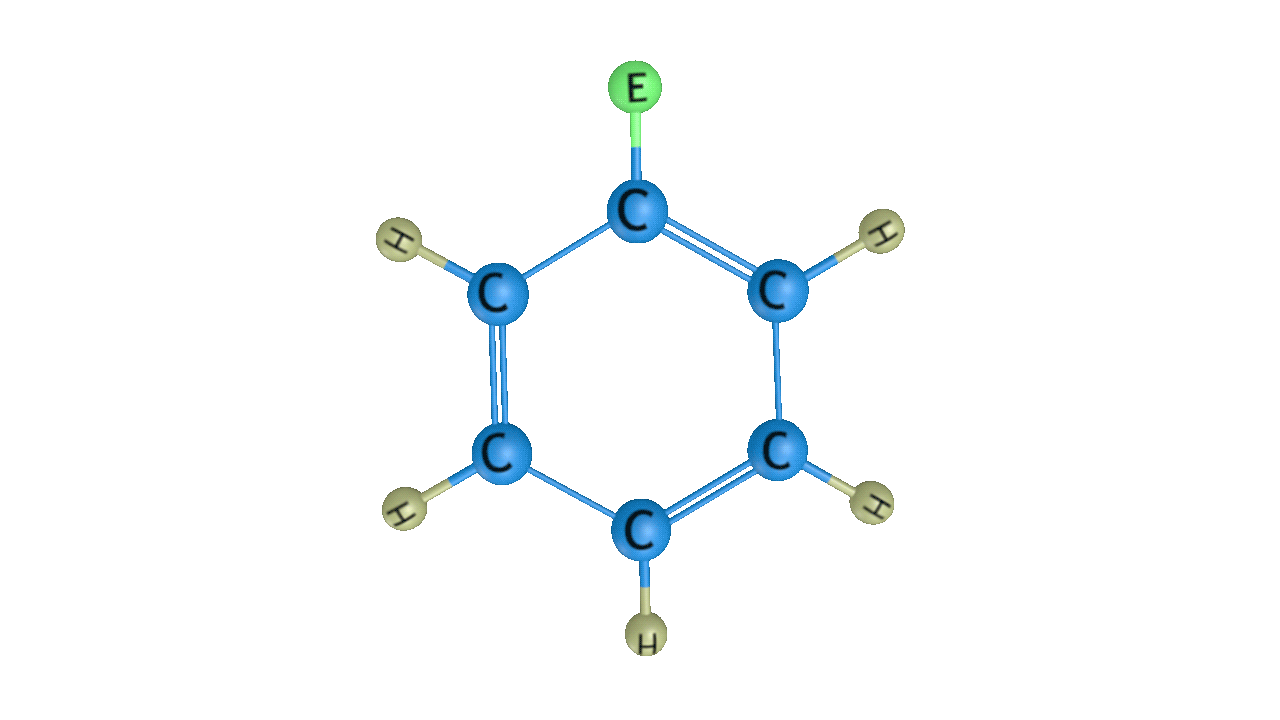
When attacked by the base, the sigma complex releases a proton from the sp3 hybridized carbon in order to restore the aromatic character, and as a result substituted product is formed. The following reaction describes the removal of a proton from the sigma complex:
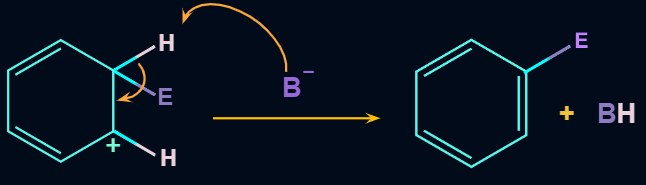
As a result, the electrophile takes the place of the hydrogen atom in the benzene ring. Because the concept of electrophilic substitution is used in many organic name reactions, it is a very important reaction in organic chemistry.
Types of Electrophilic Aromatic Substitution Reaction:
There are numerous types of electrophilic aromatic substitution reactions, the most important of which are as follows:
- Friedel-Crafts Alkylation
- Friedel-Crafts Acylation
- Electrophilic aromatic halogenations
- Aromatic nitration reactions
- Aromatic sulfonation reactions
- Aromatic mercuration reactions
- Formylation reactions
- Gattermann aldehyde reaction
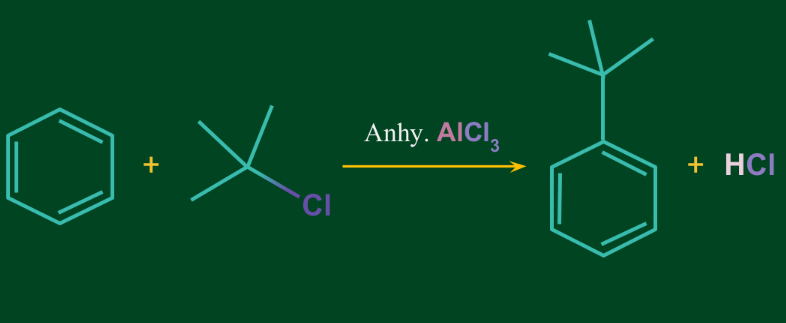
Friedel-Crafts Alkylation Reaction:
Friedel-Crafts Alkylation is a chemical reaction where the proton in the aromatic compound gets substituted with the alkyl group only. The reaction takes place in the presence of anhydrous aluminium chloride. Anhydrous aluminium chloride may also be replaced with any other Lewis acids such as Ferric chloride.
Example:
Mechanism of Friedel-Crafts Alkylation Reaction:
The mechanism of the Friedel-Crafts Alkylation reaction may be illustrated in the steps given below:
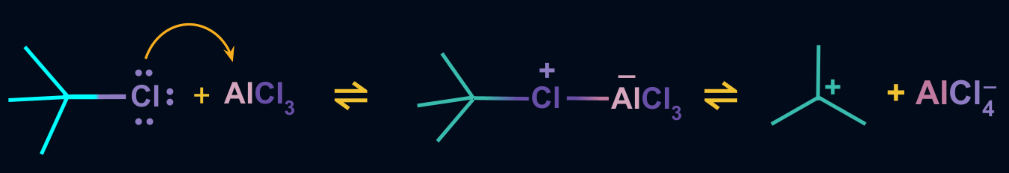
Step 1: Formation of an electrophile
The Lewis acid is taken for the reaction i.e. either anhydrous aluminium chloride or ferric chloride reacts with the alkyl halide. Generally, an electrophilic carbocation is formed as a result.
Step 2: Formation of an intermediate
The electrophilic carbocation formed by the reaction of Lewis acid and alkyl halide attacks the aromatic ring. Once it attacks the aromatic ring, an intermediate called cyclohexadienyl cation is formed. The aromatic ring loses its aromaticity because the carbon-carbon double bond breaks.
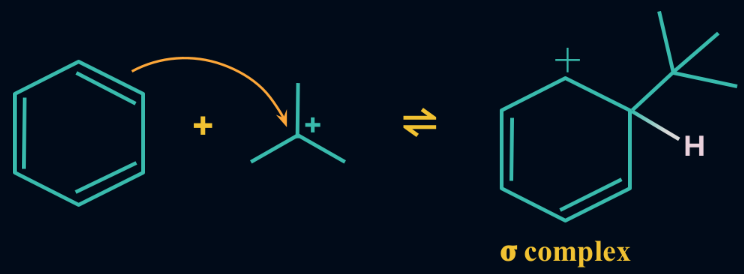
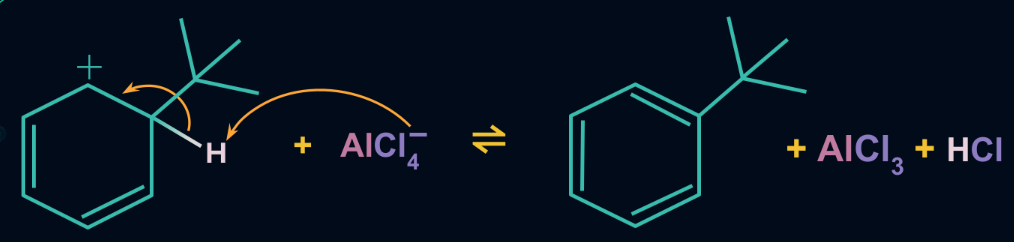
Step 3: Formation of product
The cyclohexadienyl cation undergoes deprotonation i.e. it loses one proton. The carbon-carbon double bond of the aromatic ring is reformed, thereby restoring the aromaticity of the ring too. The proton released by deprotonation regenerates the aluminium chloride catalyst.
Limitations of Friedel-Crafts alkylation reaction:
Friedel-craft’s alkylation reaction offers the following limitations.
- The carbocations formed by vinyl and aryl halides are not stable. Hence, they cannot be used in Friedel-Crafts alkylation reaction.
- Aromatic rings containing deactivating groups may not be suitable for Friedel-Crafts alkylation because deactivating groups withdraw the electron density from the benzene ring, hence the nucleophilic attack of the benzene ring will not be feasible.
- The amine group present in aniline reacts with anhydrous aluminium chloride and forms a complex which deactivates the ring. The reaction is thus incomplete.
- When benzene reacts with an alkyl halide in the presence of anhydrous lewis acid alkylation occurs on the benzene ring but polyalkylation may occur because of the activation nature of the alkyl group. To avoid this phenomenon, the aromatic sample must be taken in large quantities.
Compounds such as mono halobenzenes do not respond or participate in Friedel-Crafts alkylation reaction because they are least reactive.
Friedel-Crafts Acylation Reaction:
Friedel-Crafts acylation reaction is similar to the alkylation reaction. The only difference is that the Friedel-Crafts acylation reaction leads to the formation of a ketone, unlike the alkylation reaction.
Example:
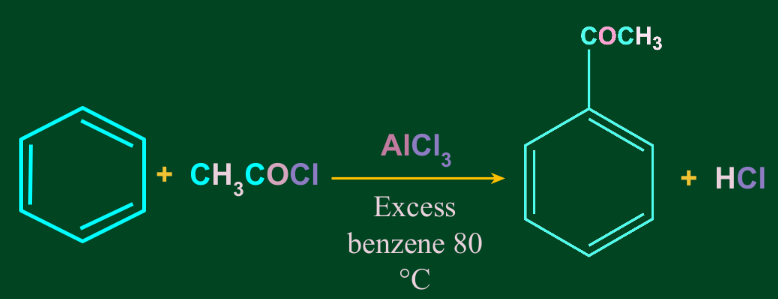
Friedel-Crafts acylation reaction is suitable for various aromatic compounds. But if the reactant is alcohol or amine, the oxygen and nitrogen atoms undergo acylation respectively.
Mechanism of Friedel-Crafts Acylation reaction:
The mechanism of the Friedel-Crafts Acylation reaction may be illustrated in the steps given below.
Step 1: Formation of acylium ion
The anhydrous aluminium chloride reacts with the acyl halide resulting in the formation of an acylium ion. The acylium ion formed is stabilized by resonance.
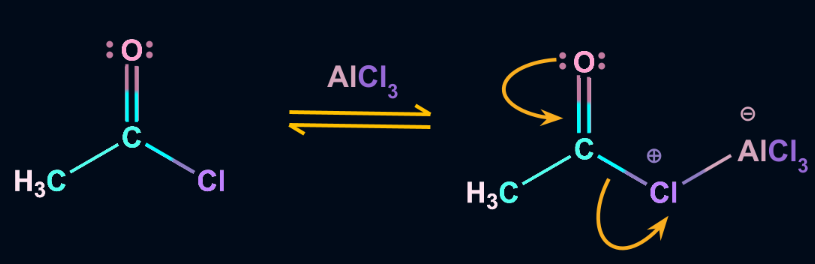

Step 2: Formation of an intermediate
The acylium ion formed by the reaction of Lewis acid and acyl halide attacks the aromatic ring. Once it attacks the aromatic ring, an intermediate is formed. The aromatic ring loses its aromaticity because the carbon-carbon double bond breaks.
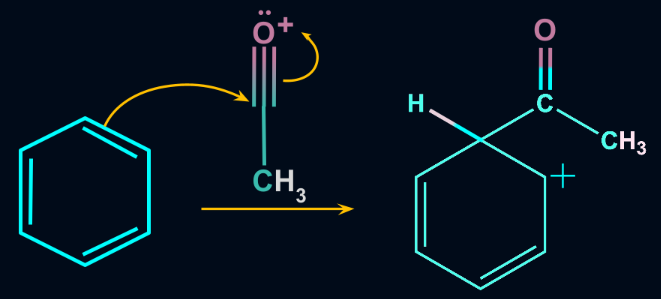
Step 3: Formation of the Product
The intermediate complex undergoes deprotonation i.e. it loses one proton. The carbon-carbon double bond of the aromatic ring is reformed, thereby restoring the aromaticity of the ring too. The proton released by deprotonation regenerates the aluminium chloride catalyst.
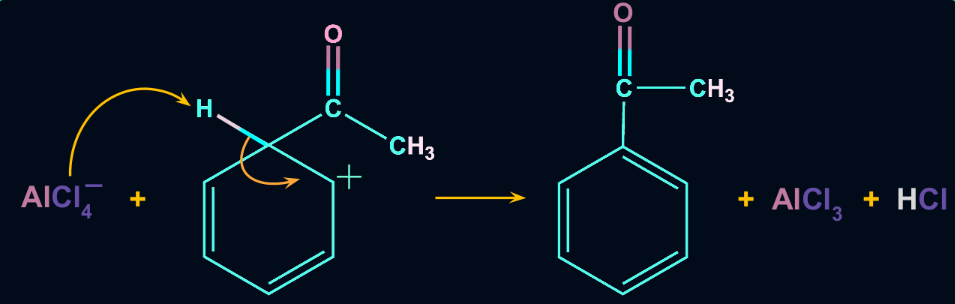
The general reactivity order of Friedel-Crafts Acylation reaction is:

Limitation of Friedel-Crafts Acylation reaction:
Friedel-craft’s acylation reaction offers the following limitations.
- Friedel-Crafts acylation reaction results in the formation of ketone products only.
- Compounds such as mono halobenzenes do not respond or participate in Friedel-Crafts acylation reaction because they are least reactive.
- Similarly, aryl amines are also not suitable for Friedel-Crafts acylation reaction because they react with Lewis acid catalyst and give rise to unreactive complexes.
Aromatic Halogenation Reaction:
Chlorination:
Benzene undergoes chlorination when it is treated with chlorine in presence of Lewis acid catalysts such as AlCl3 or FeCl3 and in absence of light.
Example:
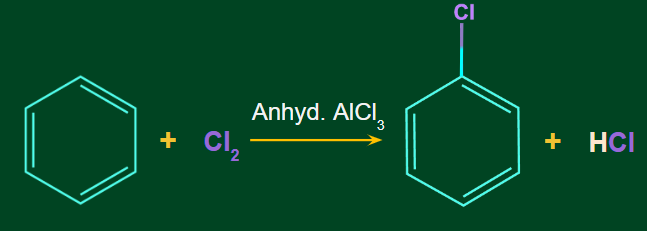
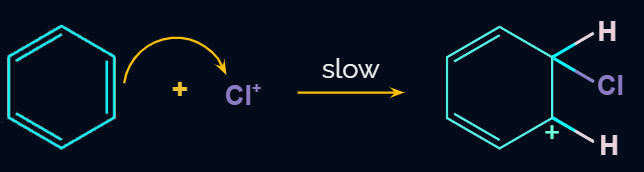
Mechanism of Chlorination:
The mechanism of the chlorination reaction may be illustrated in the steps given below.
Step 1: Formation of an electrophile


Step 2: Formation of carbocation
Electrophile (Cl+) is formed in the first step and attacks the benzene ring to form an intermediate carbocation which is resonance stabilized.
This formation of the carbocation step is a slow and rate-determining step of halogenation reaction.
The Resonance forms of carbocation are:
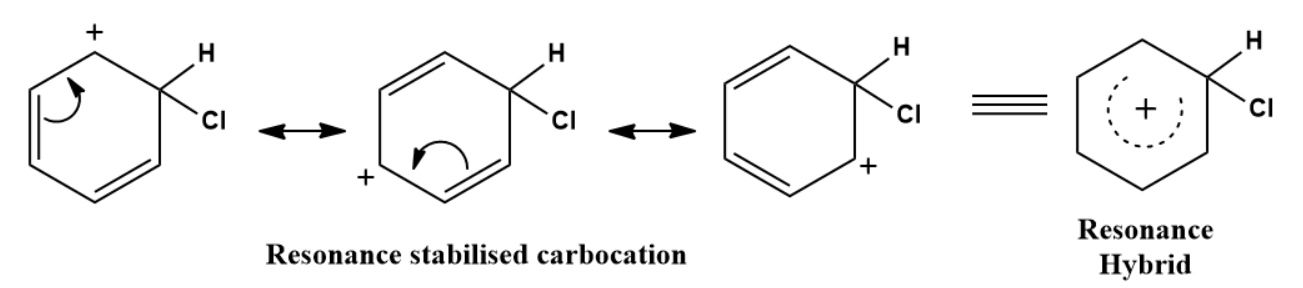
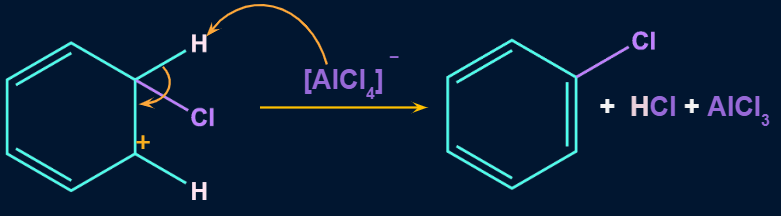
Step 3: Formation of product
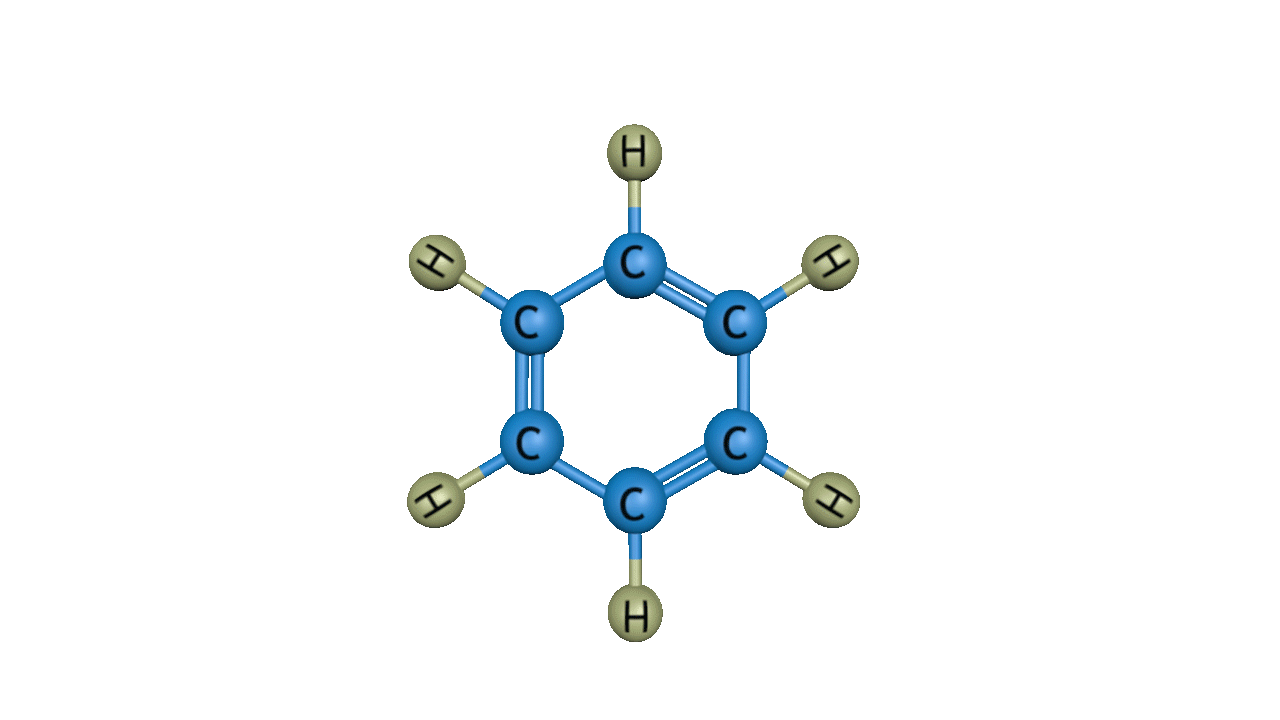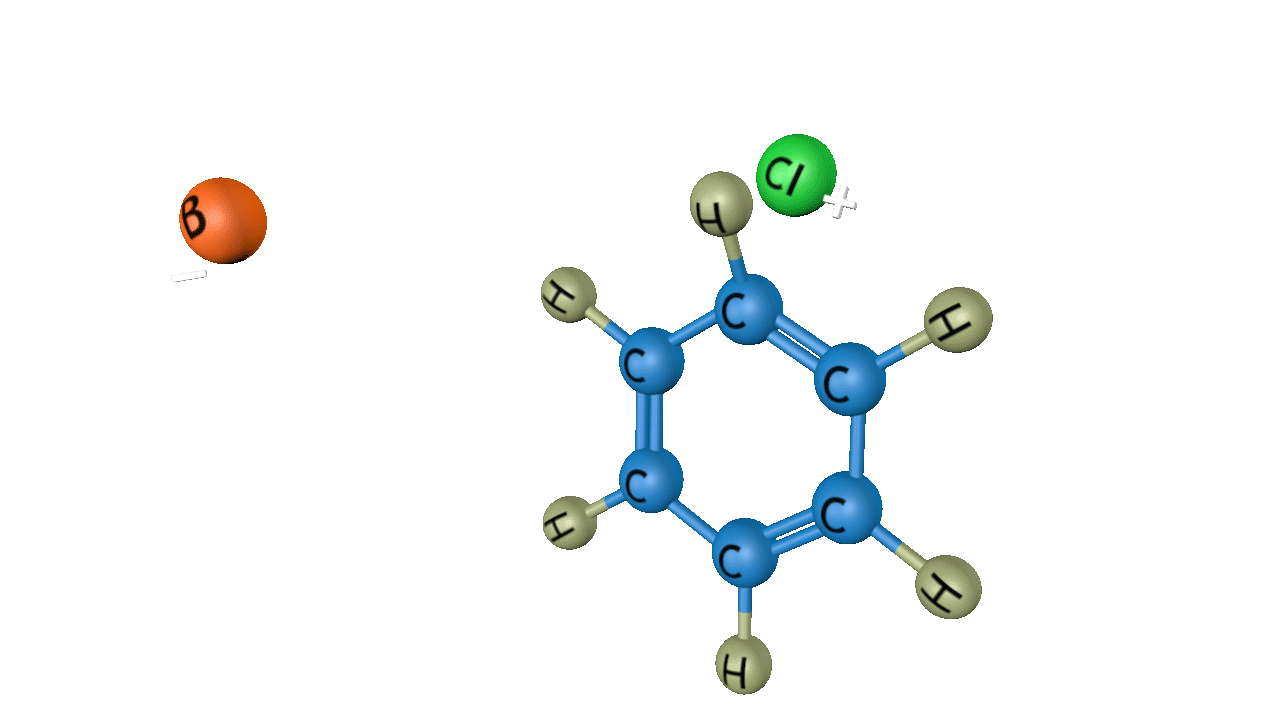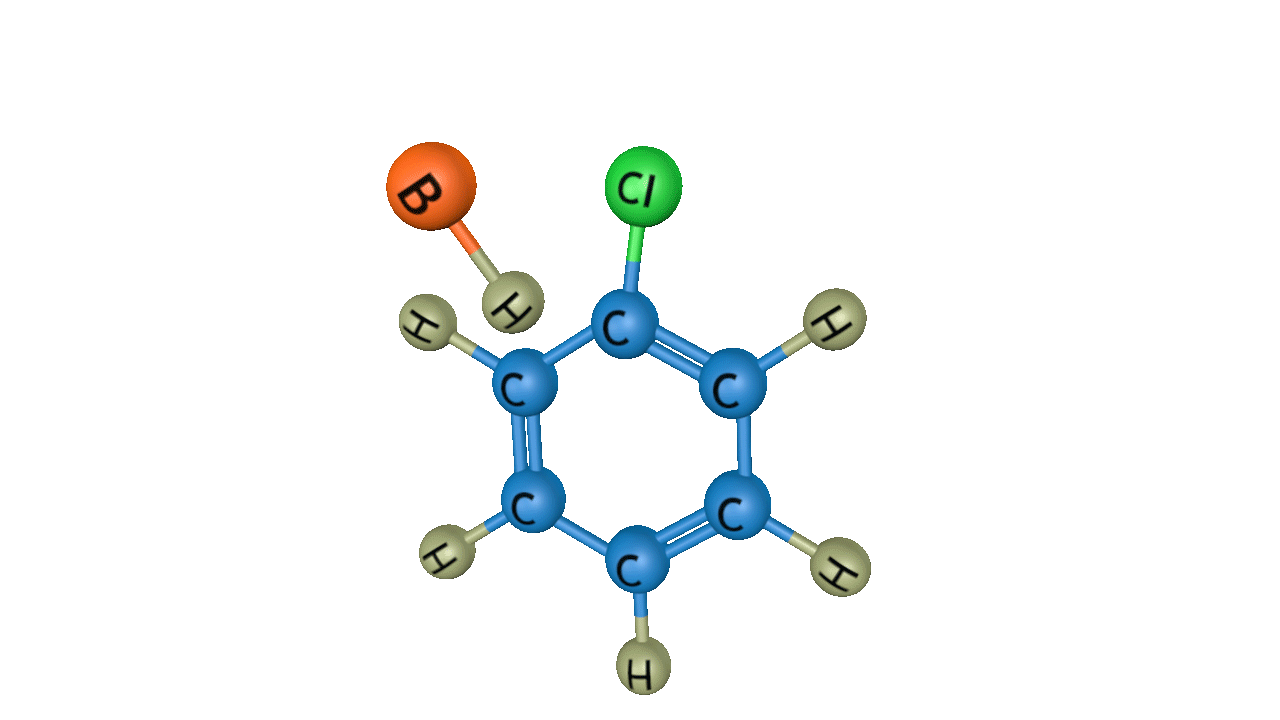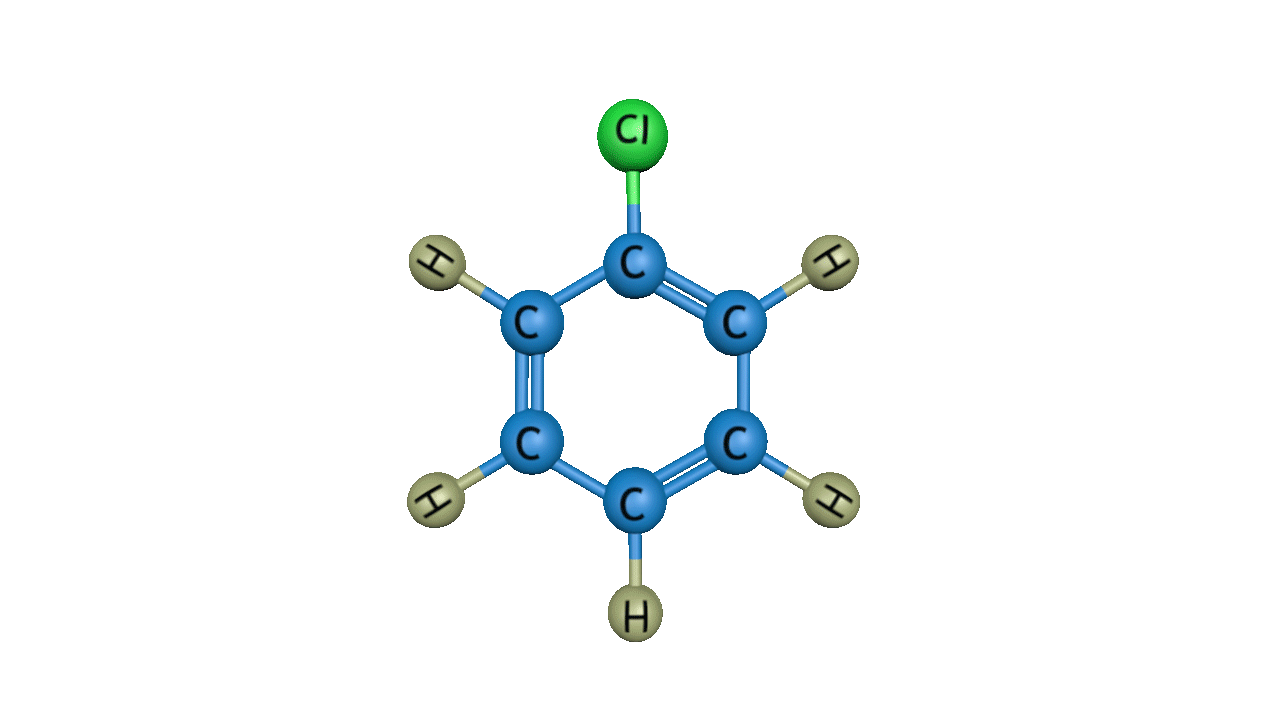
In the third step, by the attack of base the sigma complex releases a proton from the sp3 hybridized carbon in order to restore the aromatic character, and chlorobenzene is formed as a product.
Bromination:
Similarly, benzene undergoes bromination when it is treated with bromine in presence of AlBr3 or FeBr3, but not as fast as chlorination.
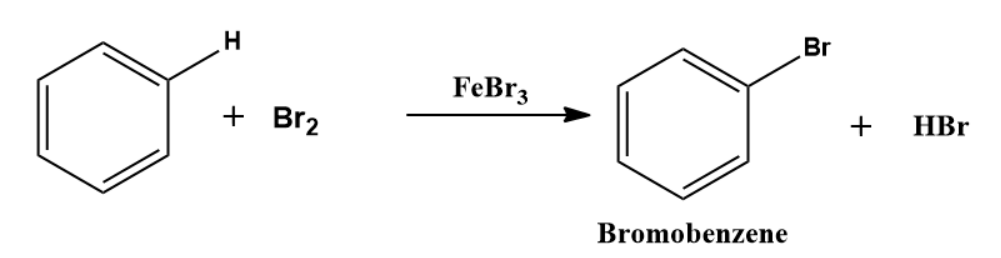
Mechanism of Bromination:
The mechanism of the bromination reaction may be illustrated in the steps given below.
Step 1: Formation of an electrophile

Step 2: Formation of carbocation
Electrophile is formed in the first step and attacks the benzene ring to form an intermediate carbocation which is resonance stabilized.
This formation of the carbocation step is a slow and rate-determining step of sulphonation reaction.
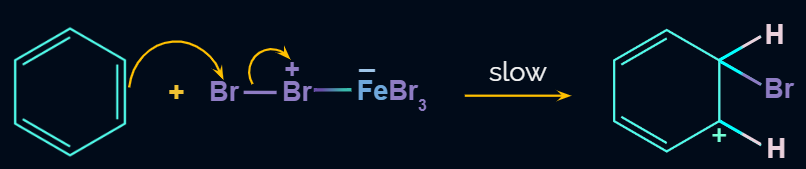
Step 3: Formation of product
In the third step, by the attack of base the sigma complex releases a proton from the sp3 hybridized carbon in order to restore the aromatic character, and chlorobenzene is formed as a product.
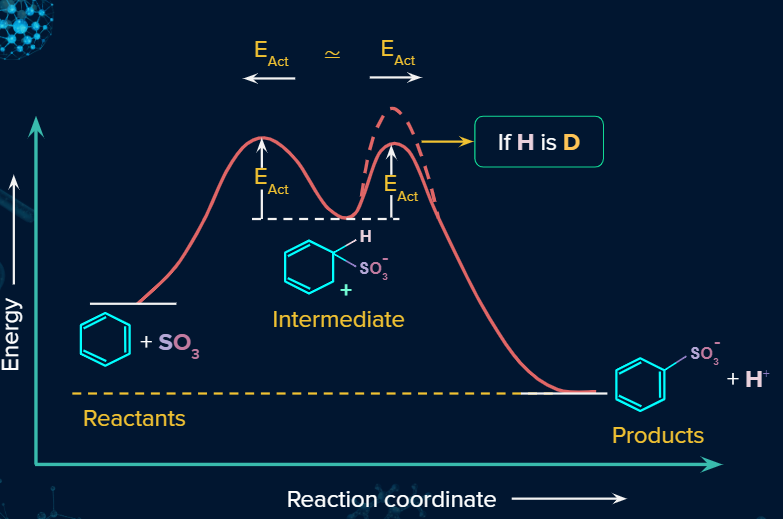
Energy Profile diagram for Bromination of Benzene:
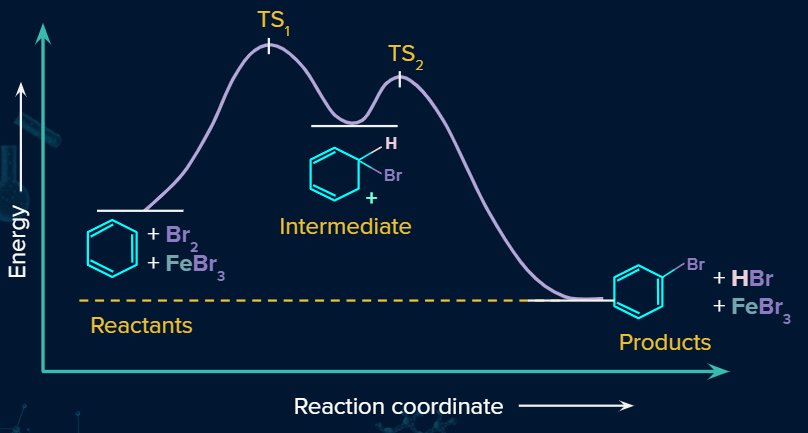
Iodination:
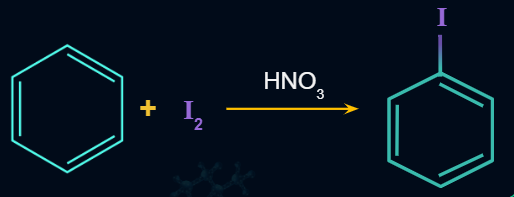
Iodination of Benzene is a slow and reversible process. It takes place in the presence of an oxidizing agent i.e., HIO3,HNO3 or HgO to remove formed HI which is a strong reducing agent.
Fluorination:
Fluorination of benzene is not possible because an explosion takes place. Fluorine reacts so rapidly with benzene such that aromatic fluorination requires special conditions and apparatus. Even then, it is difficult to limit the reaction to monofluorination.
C6H6 +3F2 6HF +6C
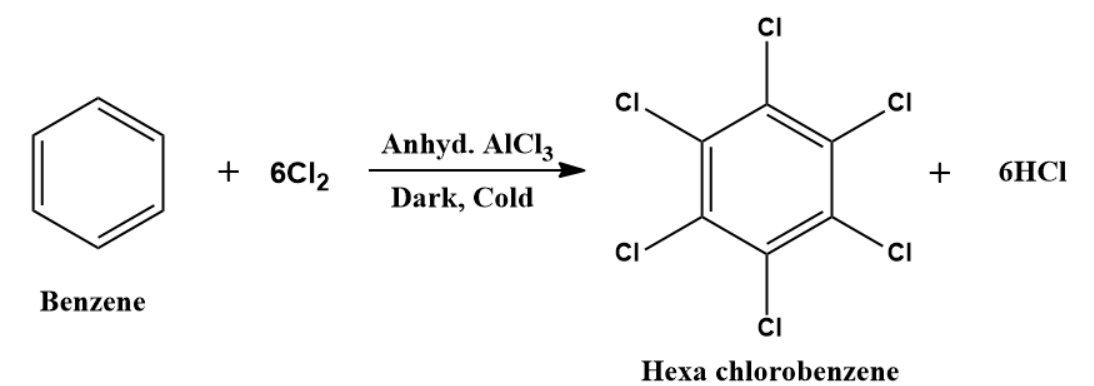
With an excess of halogens in presence of anhydrous AlCl3 (catalyst) and dark, all the hydrogen atoms of the benzene ring may be successively replaced.
Example:
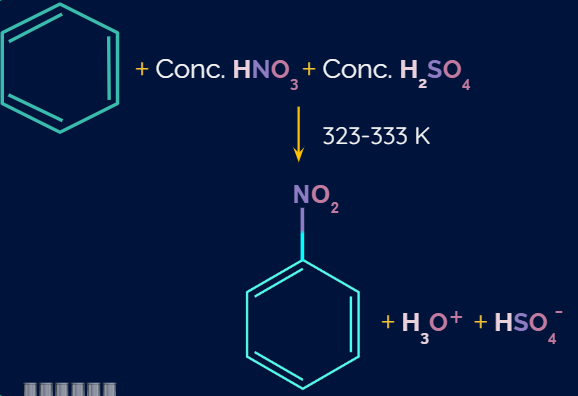
Aromatic Nitration Reaction:
When benzene is treated with concentrated nitric acid in the presence of concentrated sulphuric acid, a hydrogen atom of benzene ring is replaced by a nitro group, and nitrobenzene is formed as a product. This reaction is carried out at 40-50C temperature.
Example:
Mechanism of Nitration:
The mechanism of the nitration reaction may be illustrated in the steps given below.
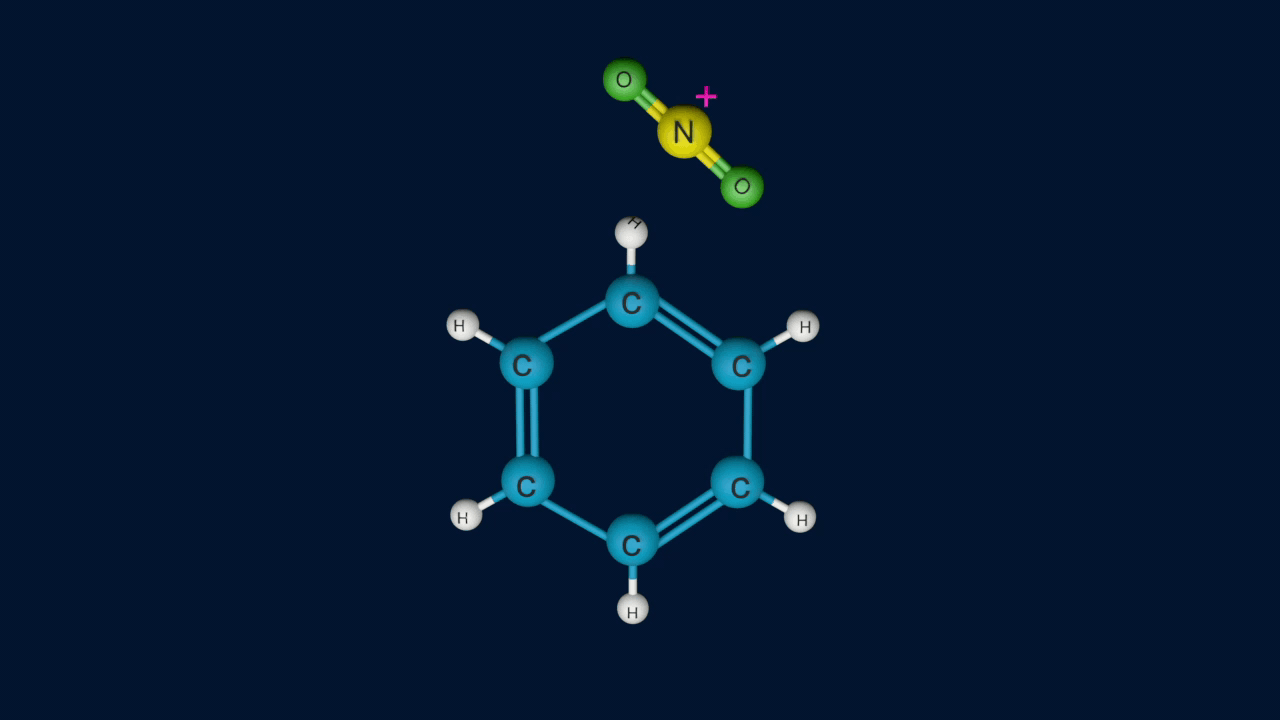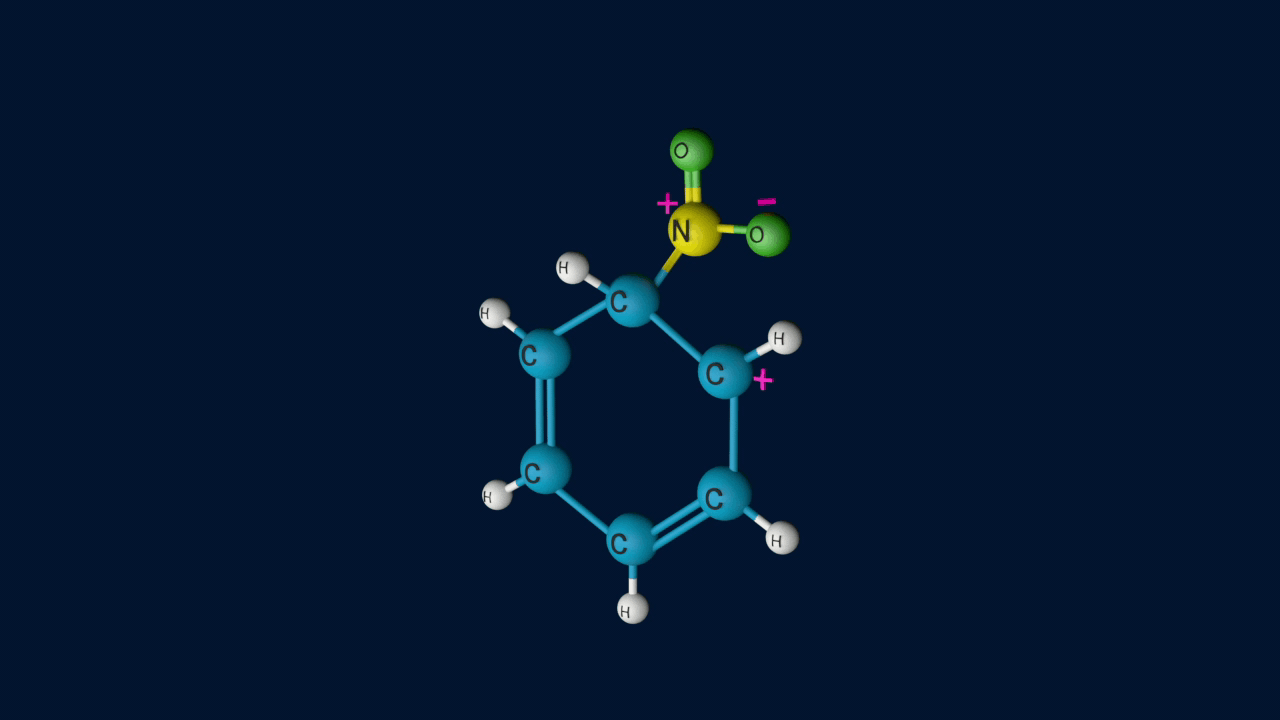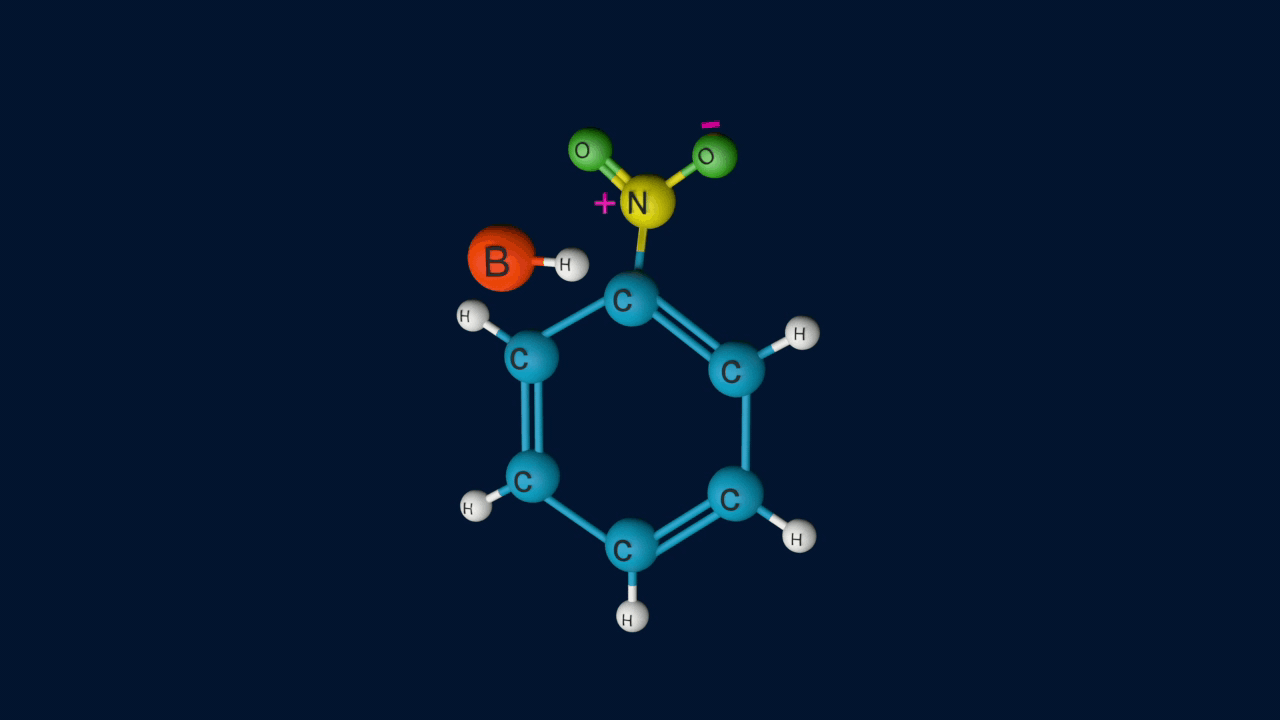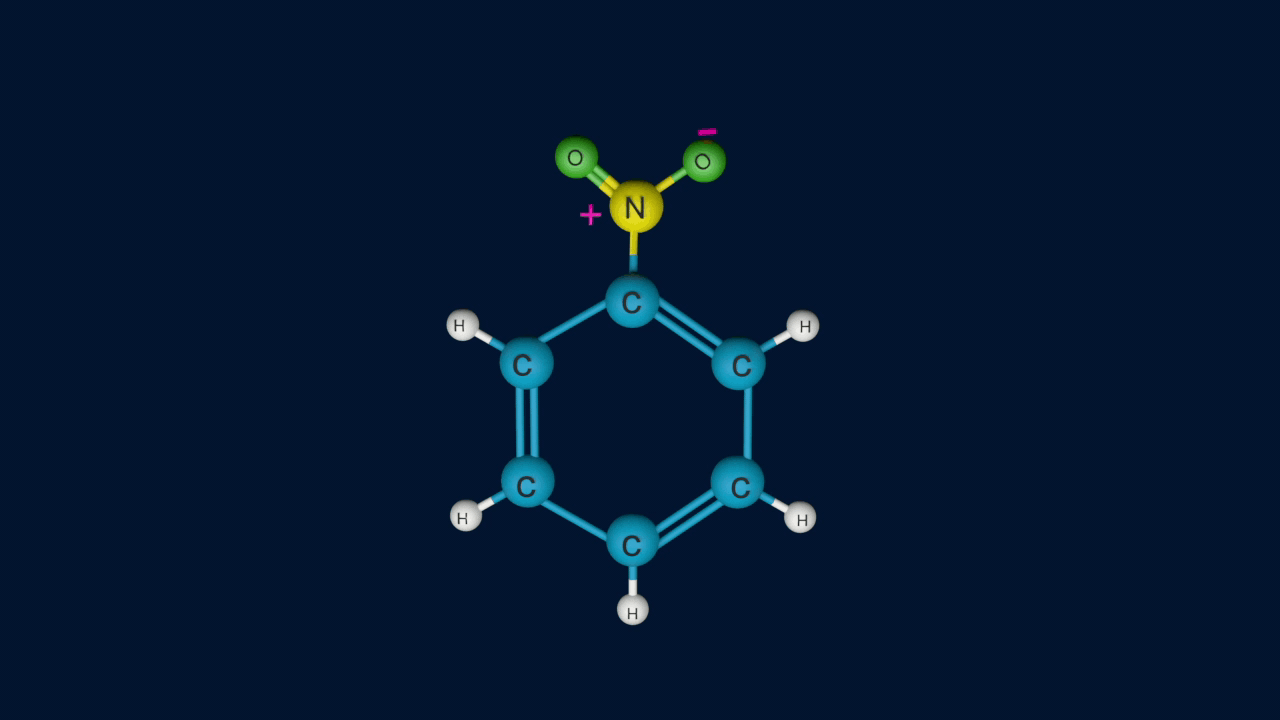
Step 1: Formation of an electrophile
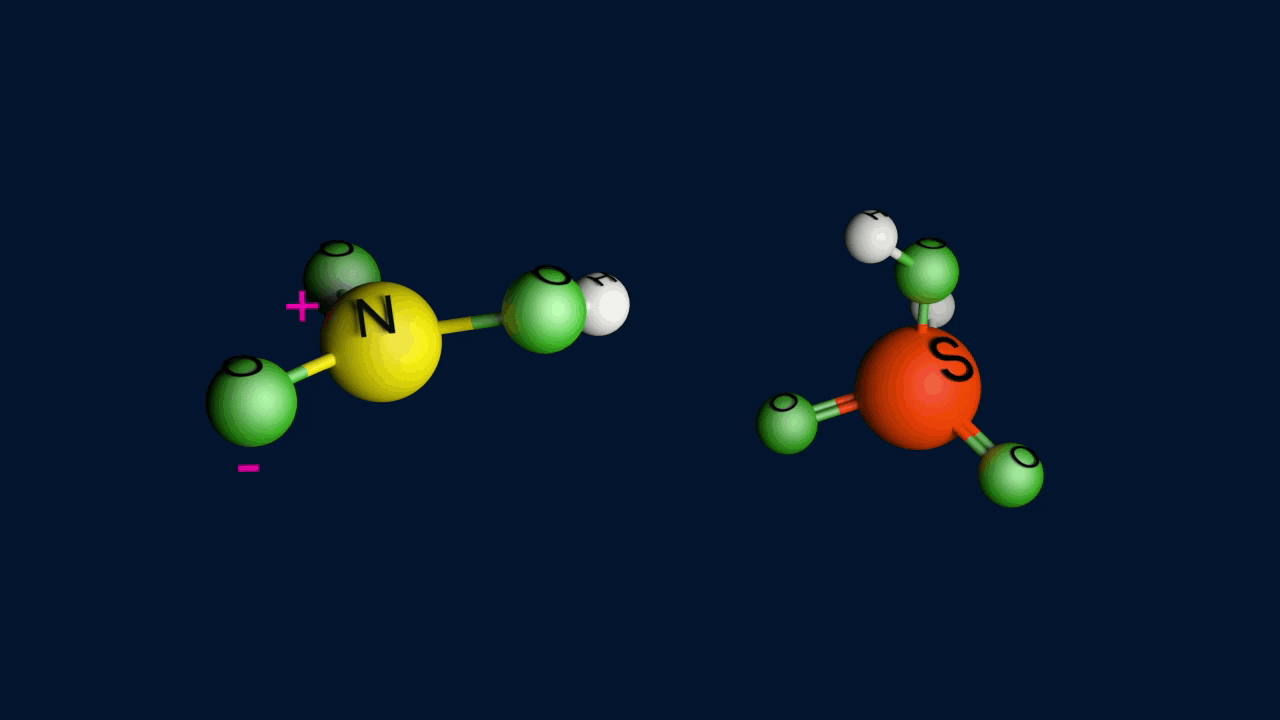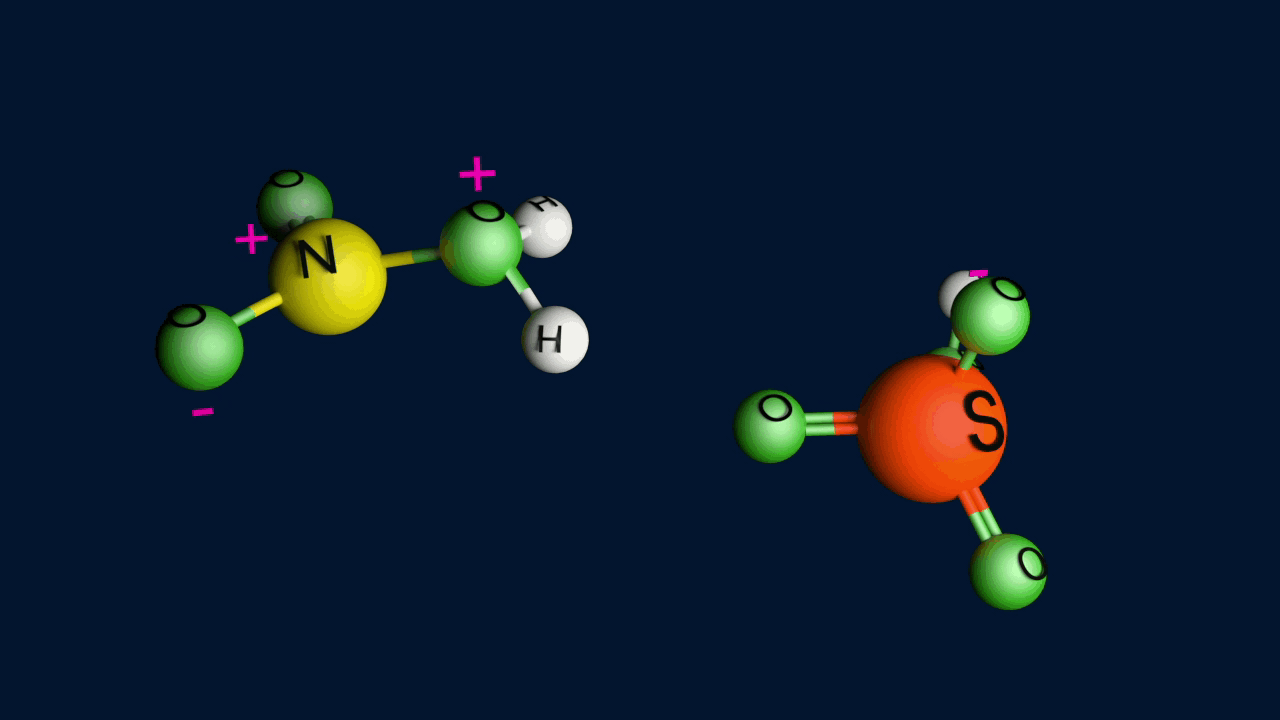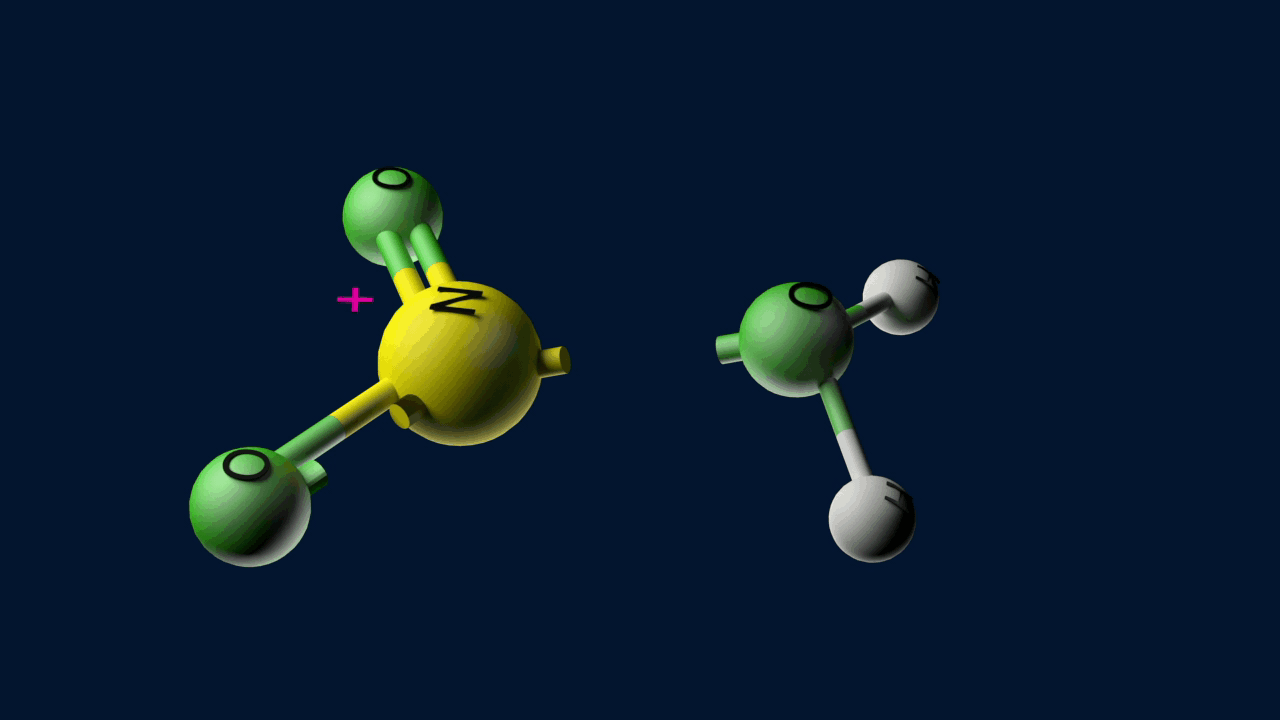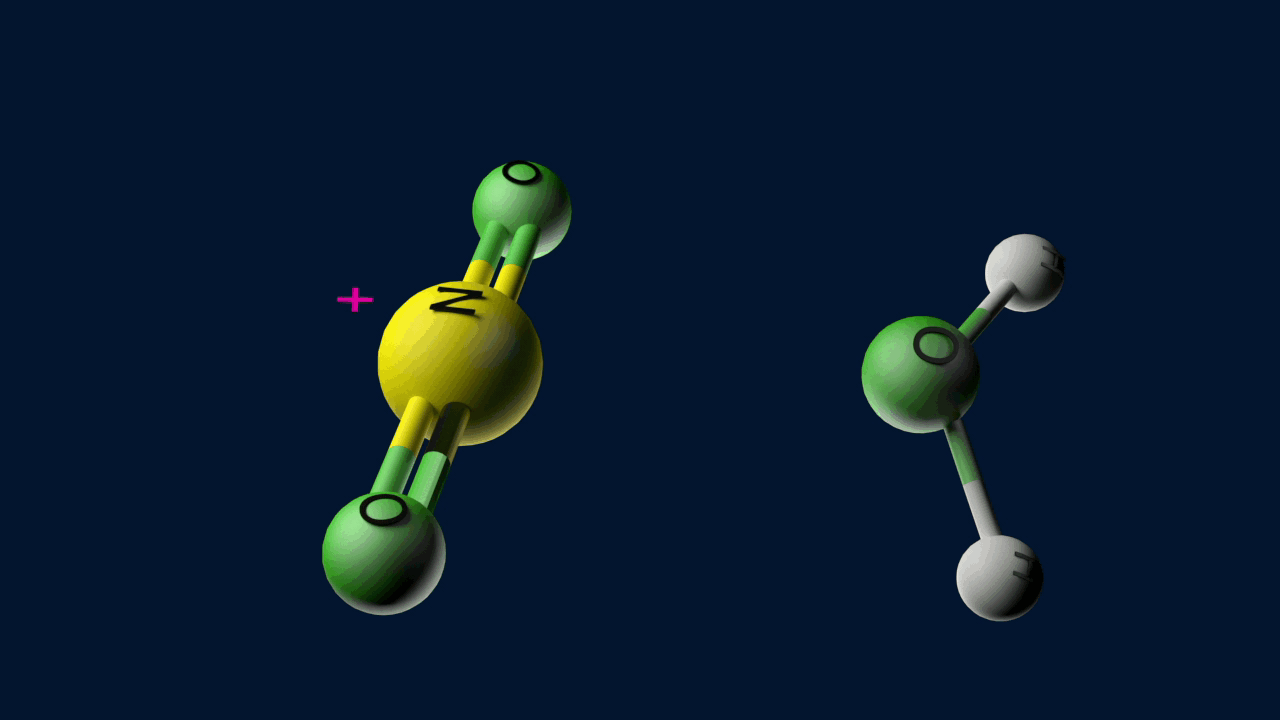
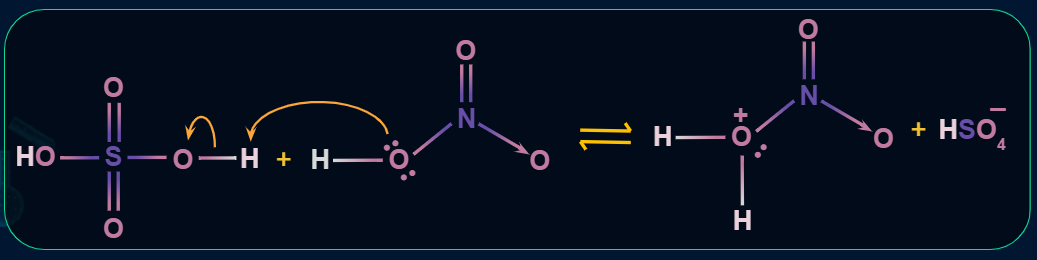
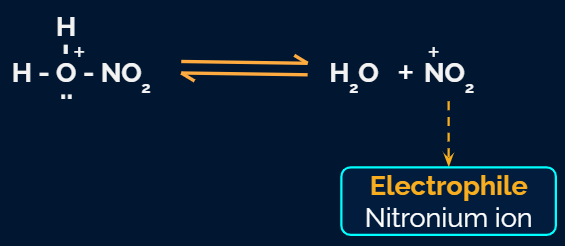
Step 2: Formation of carbocation
Electrophile (NO2+) is formed in the first step and attacks the benzene ring to form an intermediate carbocation which is resonance stabilized.
This formation of the carbocation step is a slow and rate-determining step of nitration reaction.
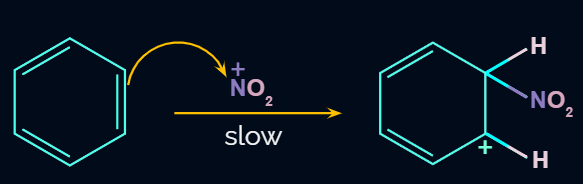
The Resonance forms of carbocation are:
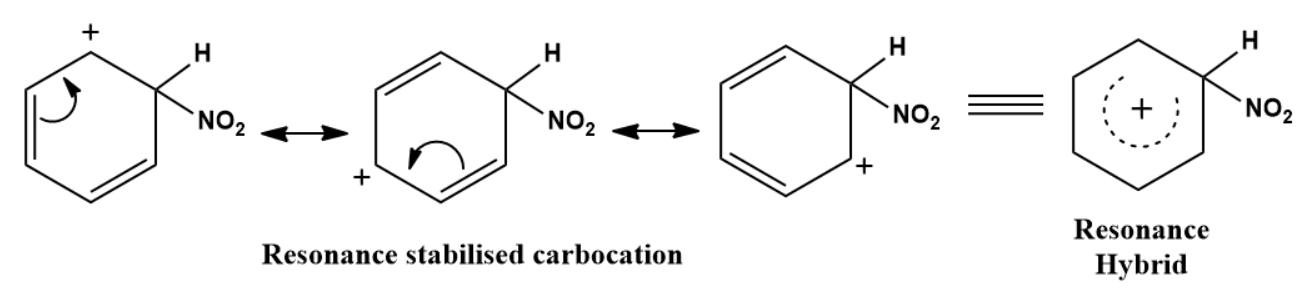
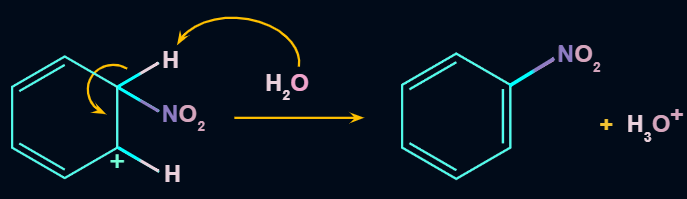
Step 3: Formation of product
In the third step, by the attack of nucleophile the sigma complex releases a proton from the sp3 hybridized carbon in order to restore the aromatic character, and nitrobenzene is formed as a product.
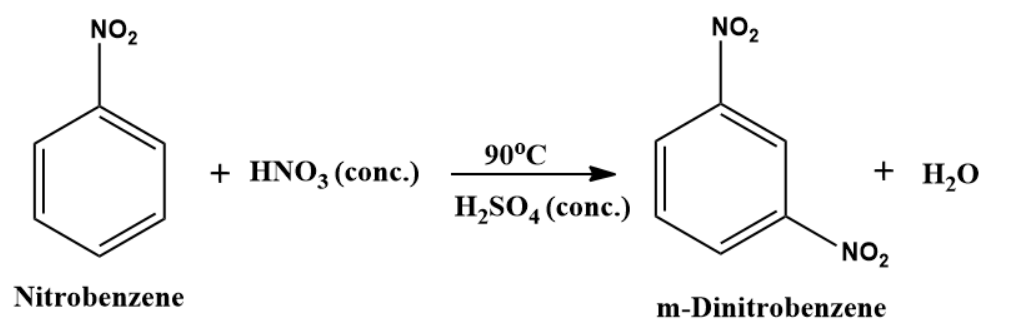
If nitrobenzene is further nitrated or benzene is treated with a nitrating mixture at 90c m-Dinitrobenzene is formed as a product.
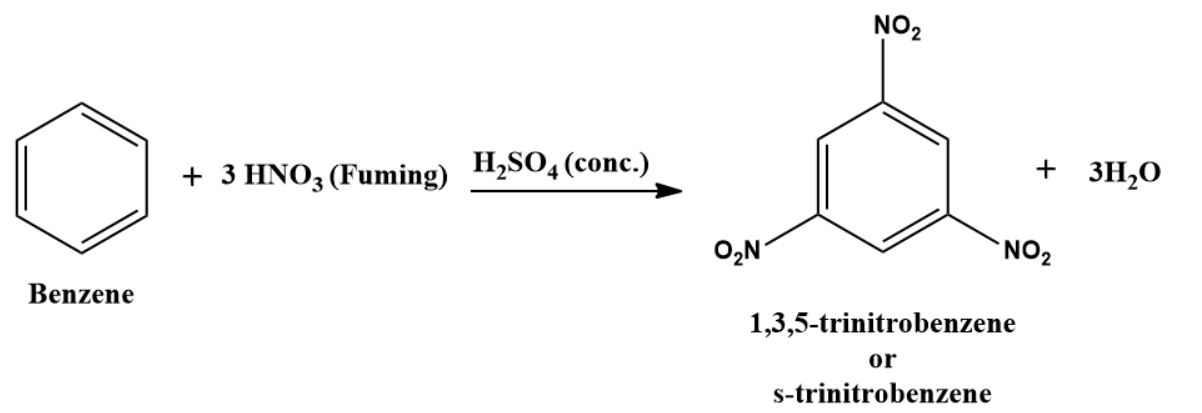
If Benzene is treated with fuming nitric acid and concentrated sulphuric acid, 1,3,5-trinitrobenzene is formed as a product that is used as a powerful explosive.
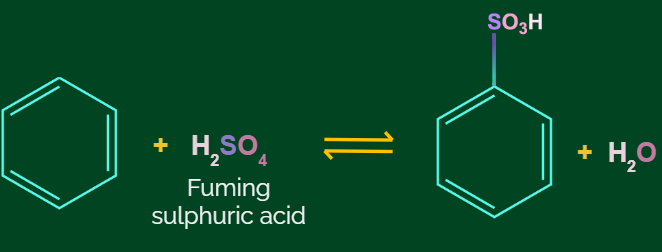
Aromatic Sulfonation Reaction:
When benzene is treated with hot concentrated sulphuric acid, a hydrogen atom of benzene ring is replaced by a sulfonic acid group, and benzene sulphonic acid is formed as a product.
This reaction is known as the Sulphonation reaction.
Example:
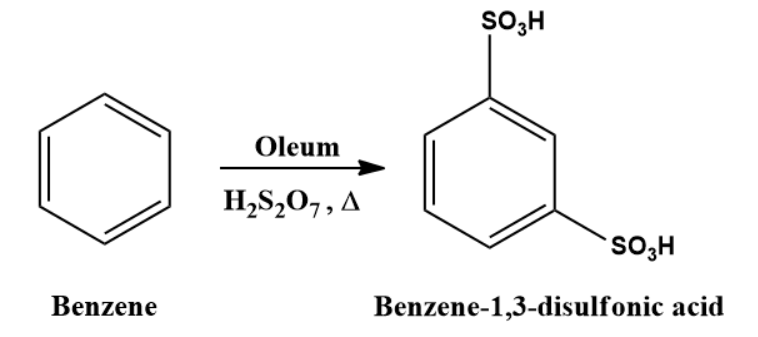
When benzene is heated at a high temperature in the presence of oleum, m-Benzenedisulphonic acid is formed.
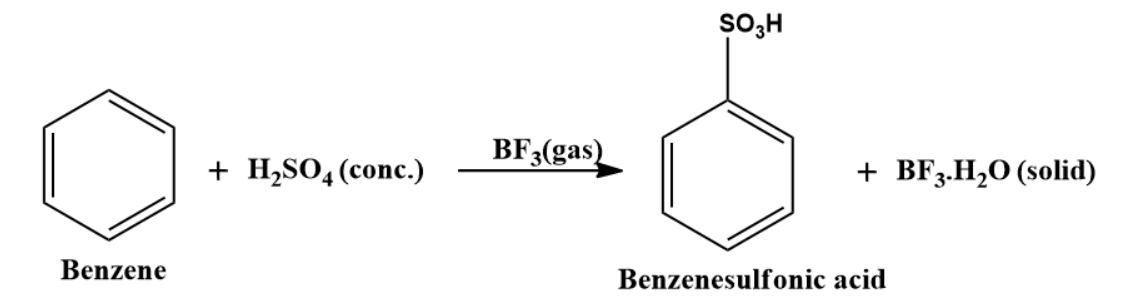
Sulphonation of benzene can also be carried out at room temperature in the presence of BF3 as catalyst.
Mechanism of Sulphonation:
The mechanism of the sulfonation reaction may be illustrated in the steps given below.
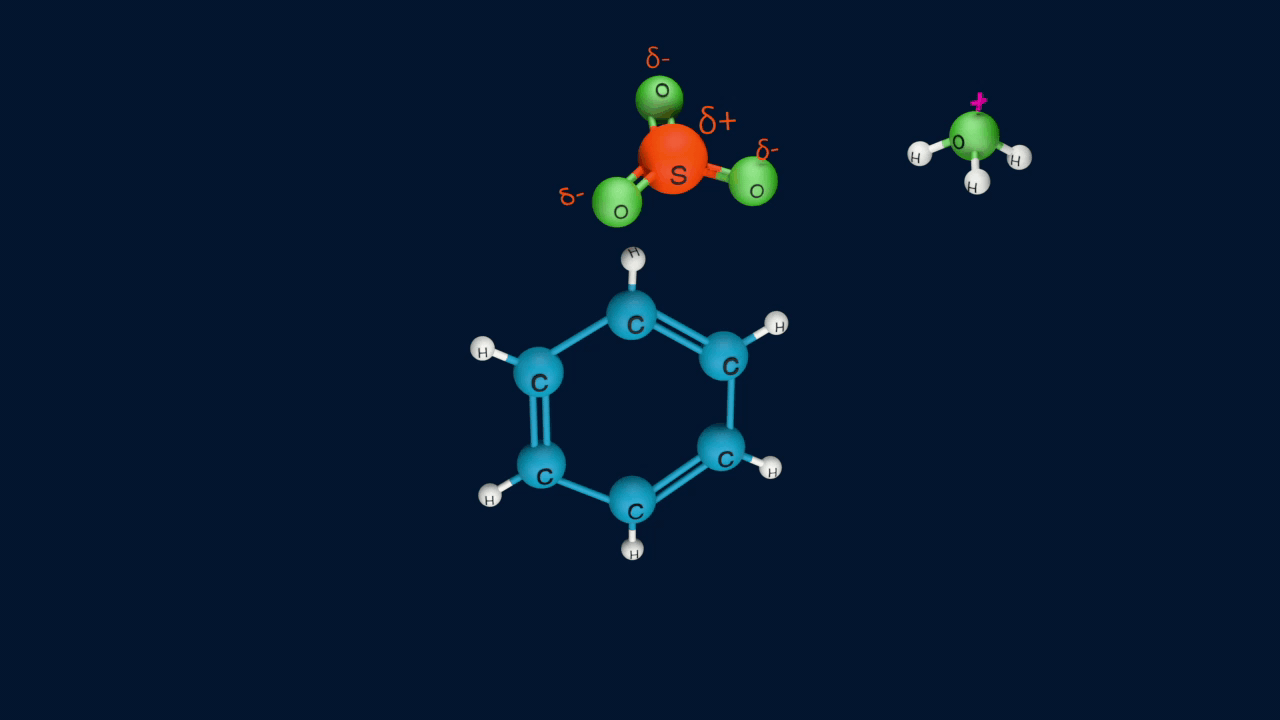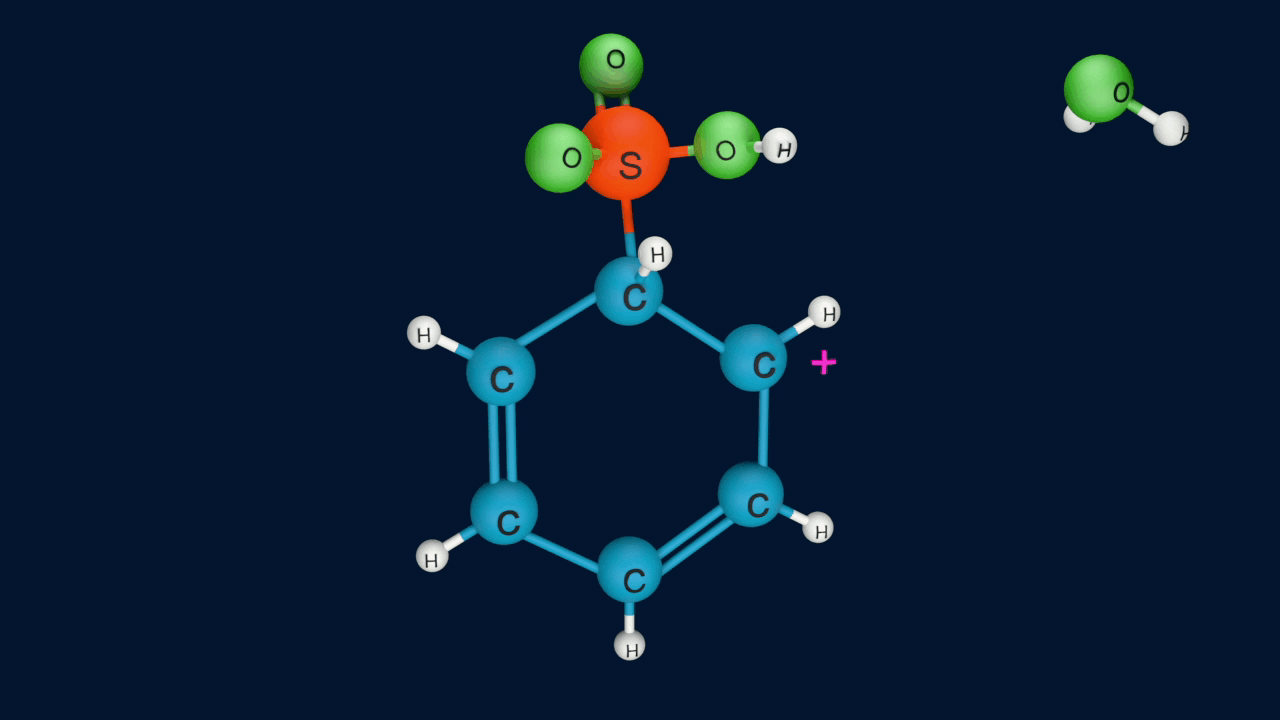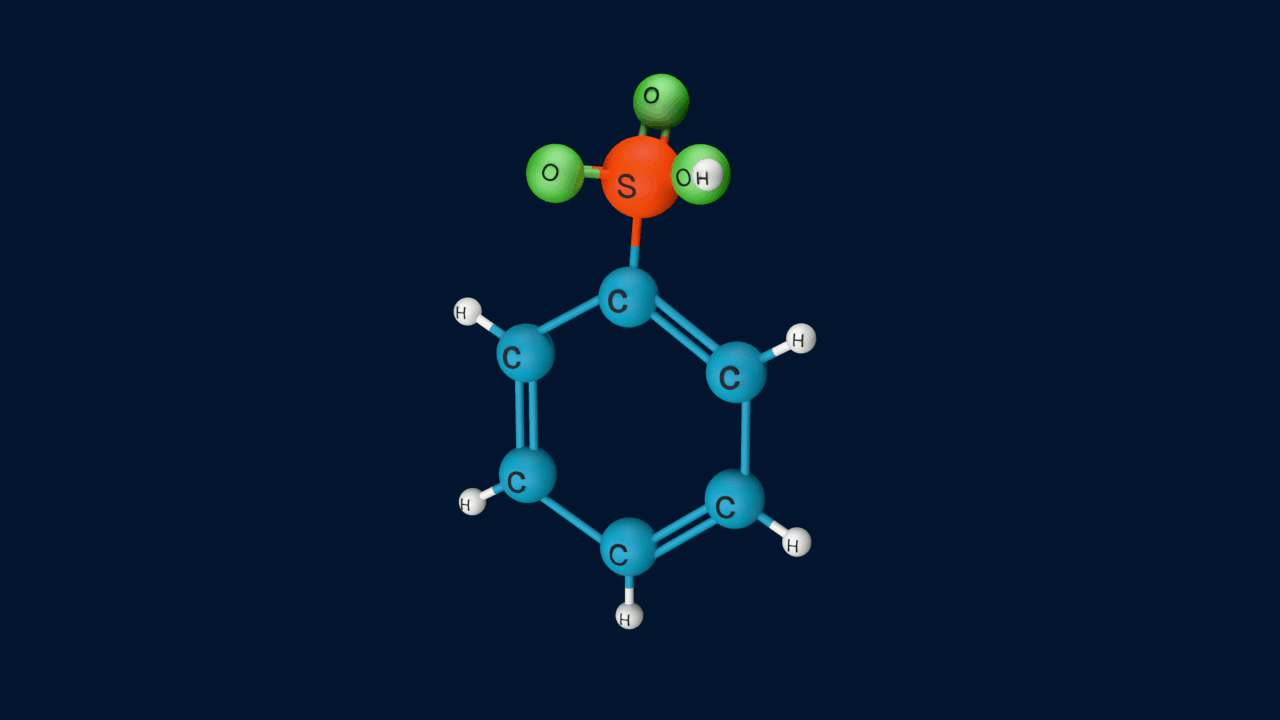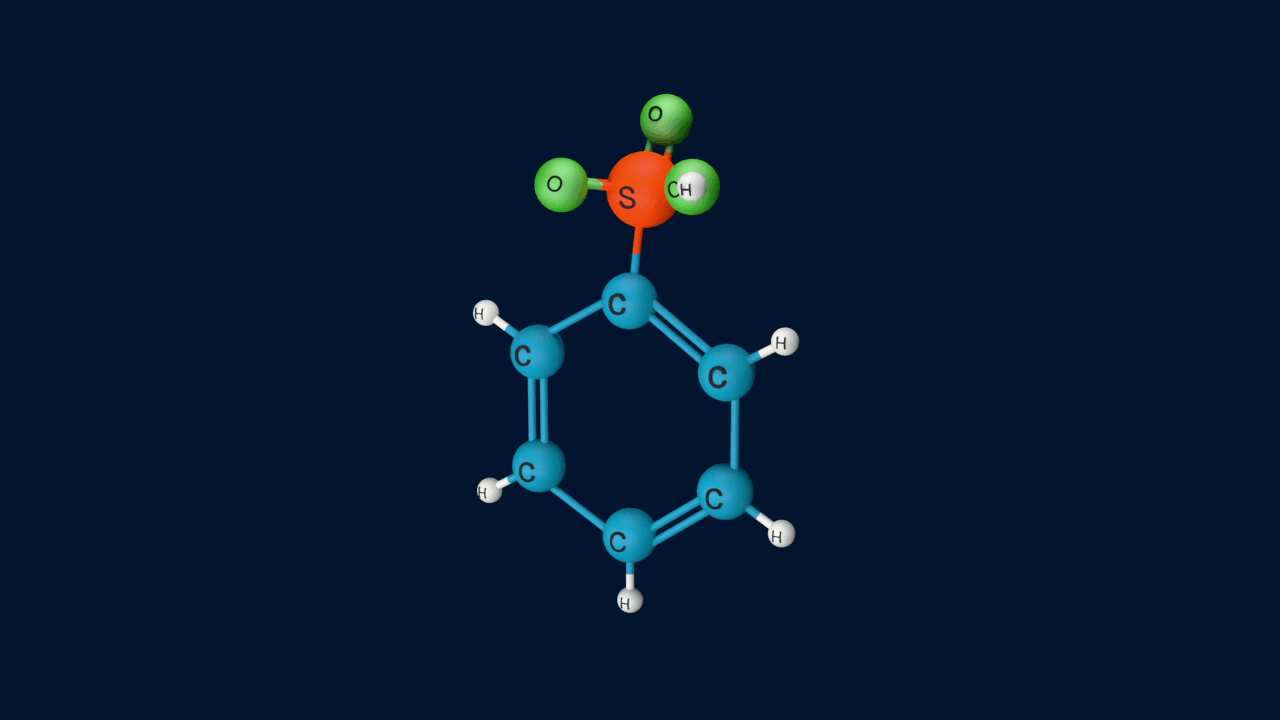
Step 1: Formation of an electrophile
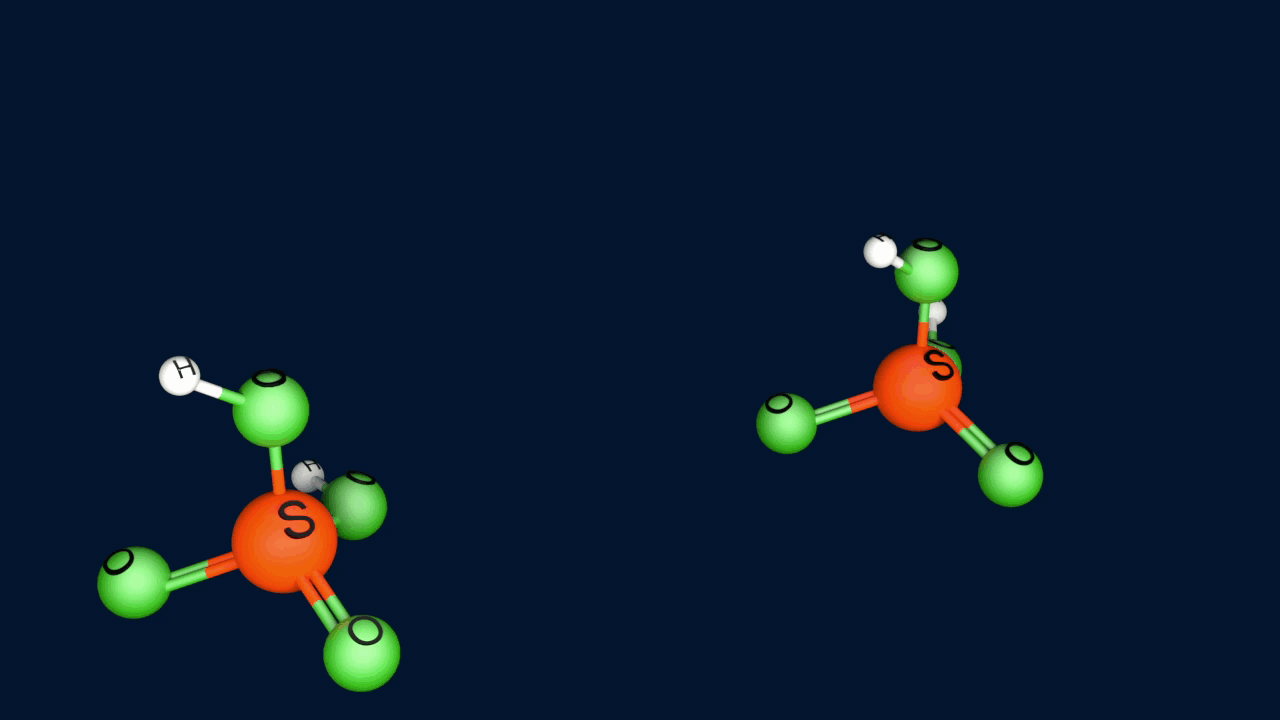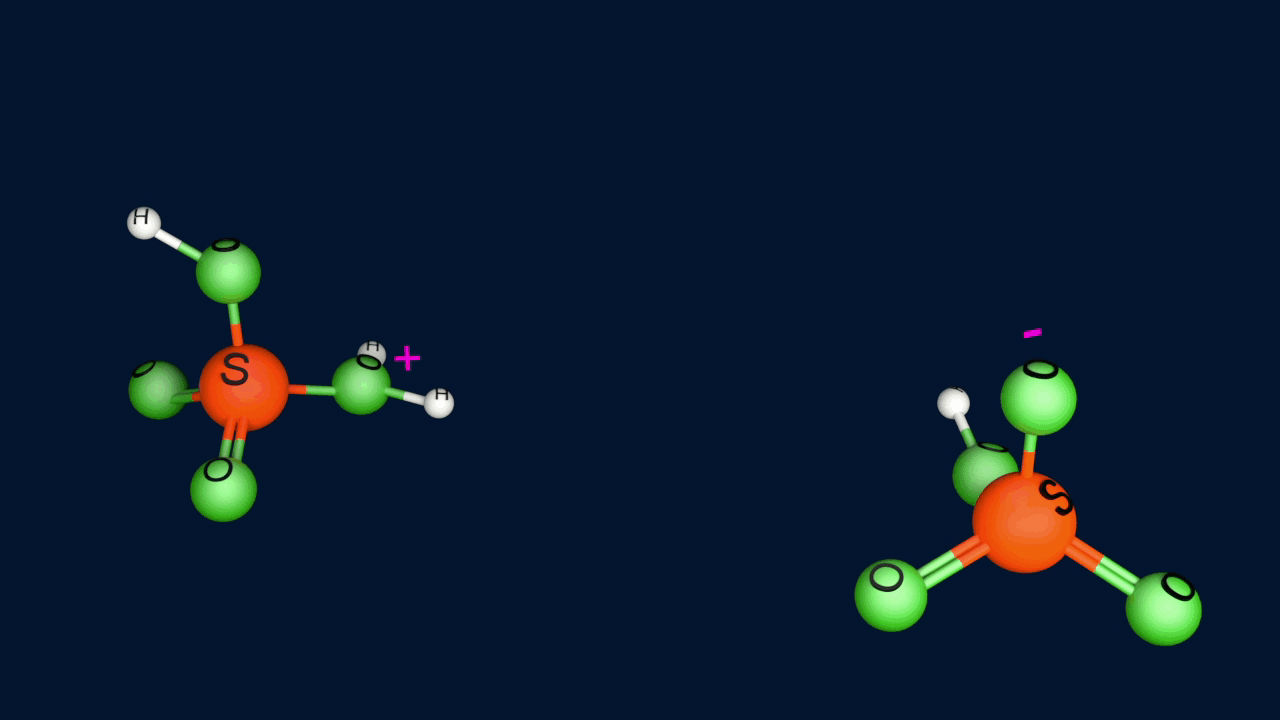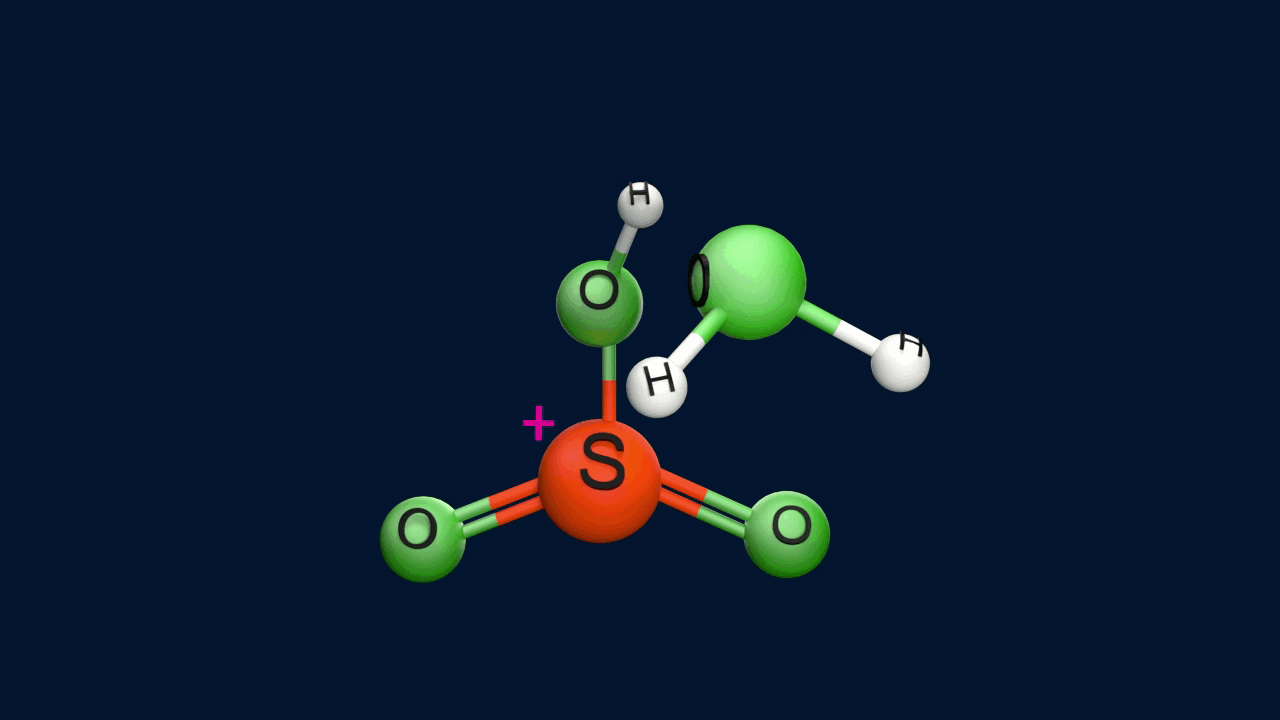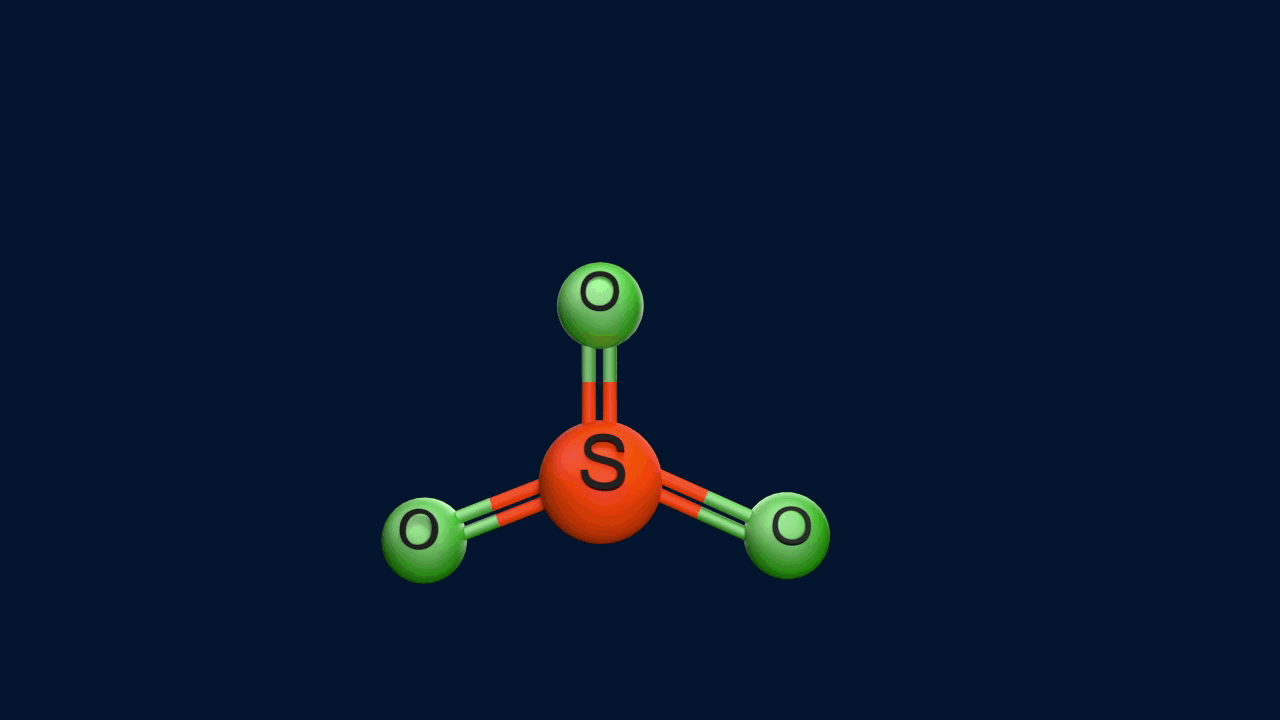
|
2H2SO4 ⇌ H3O+ + HSO4- + SO3 |
Step 2: Formation of carbocation
Electrophile (SO3) is formed in the first step and attacks the benzene ring to form an intermediate carbocation on which is resonance stabilized.
This formation of the carbocation step is a slow and rate-determining step of sulphonation reaction.
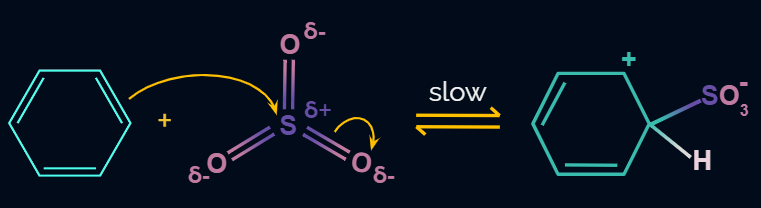
The Resonance forms of carbocation are:
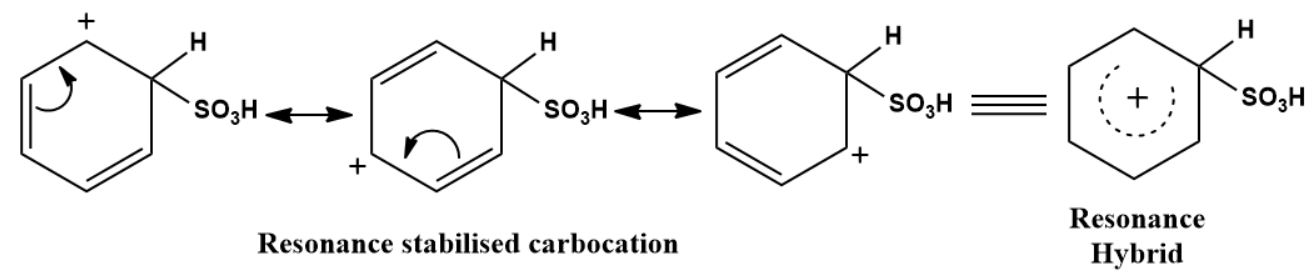
Step 3: Removal of proton
In the third step, by the attack of base the sigma complex releases a proton from the sp3 hybridized carbon in order to restore the aromatic character.
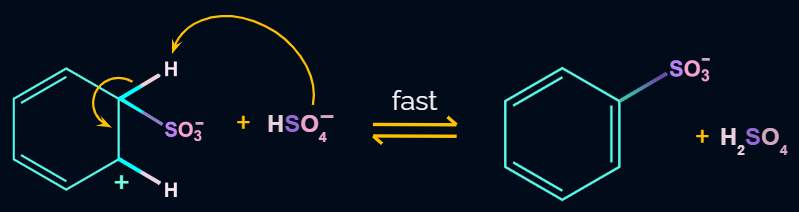
Step 4: Formation of Product
In the last step the anion formed in the third step undergoes hydrolysis to yield benzene sulphonic acid as a product.
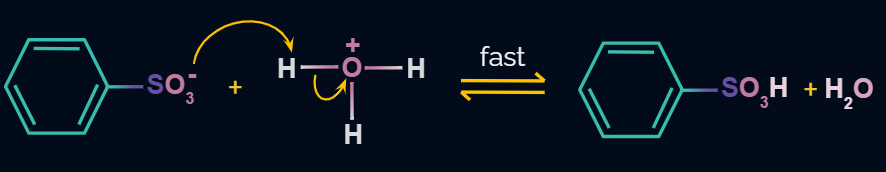
Energy Profile Diagram:
Practice Problems:
Q1. Although chlorine is an electron-withdrawing group, then what will be directive influence?
(A) ortho
(B) meta
(C) ortho-para
(D) none of the above
Answer: C
Solution:
The lone pair of the chlorine shows +M effect and increases the electron density over the ortho and para positions hence it is considered as an ortho-para directing group.
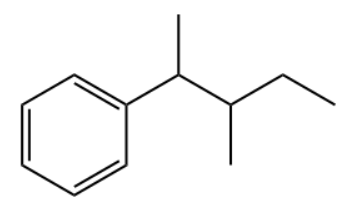
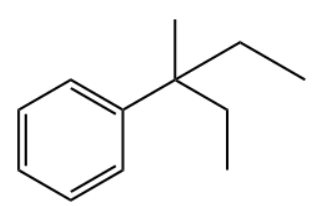
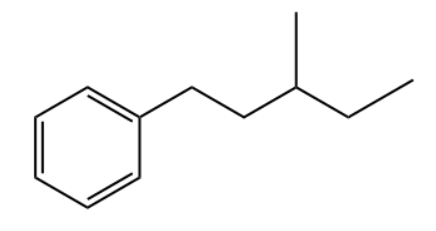
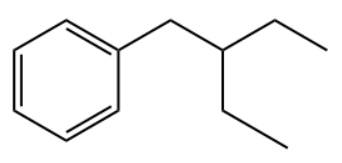
Q2. Which of the following is the primary product of the given reaction?
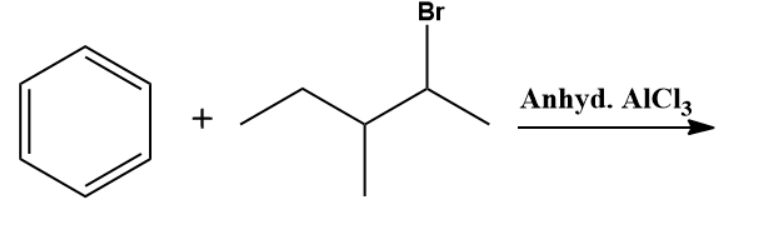
Answer: B
Solution: This is a Friedel-craft alkylation reaction that uses alkyl halides as reactant in the presence of anhydrous aluminium chloride to form alkylbenzenes.
This is accomplished through an electrophilic attack on the aromatic ring using a carbocation. The formed secondary carbocation will rearrange into a tertiary carbocation.
So, the correct option is B.
Q3. What is the outcome of the following reaction?
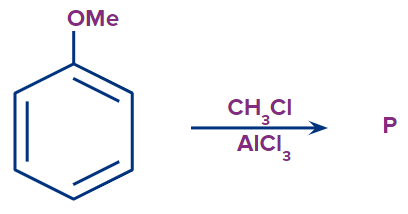
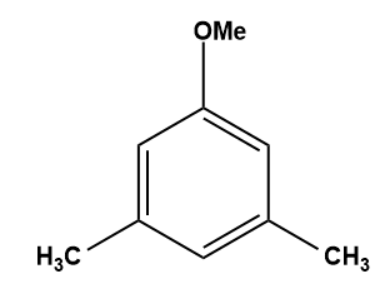
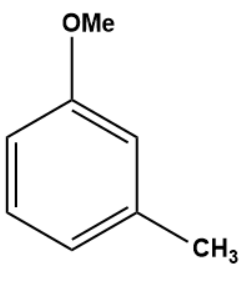
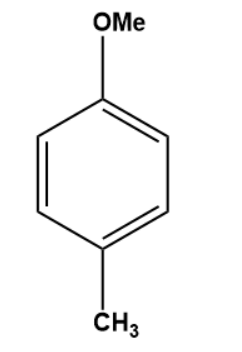
- Both A and B
Answer: C
Solution: The given reaction is an electrophilic substitution reaction called a Friedel-craft alkylation. The ortho and para directing groups are the groups that direct the incoming electrophile to ortho and para positions. The density of electrons increases with ortho and para position. As a result, substitution occurs primarily on ortho and para positions.
The ortho-para directing group is -OMe.
As a result, C is the correct answer.
Q4. The compound X in the reaction is :

Answer: B
Solution:
ICl with anhydrous AlCl3 will generate I+ ion which can undergo iodination easily. So, the product
formed will be iodobenzene. Chloride is present as a nucleophile. Thus, it can not show an electrophilic
aromatic substitution reaction.
Mechanism:
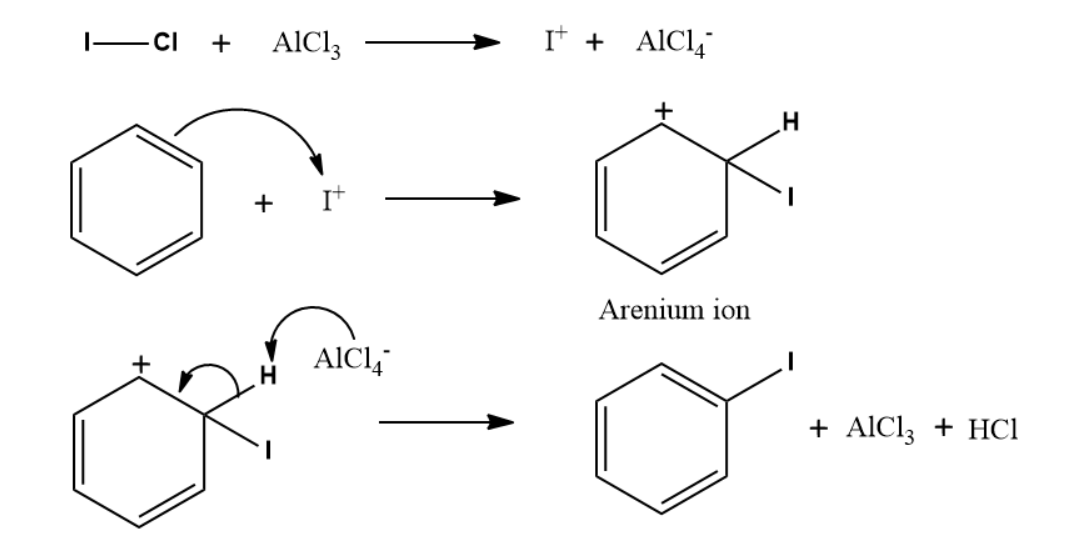
So, the correct option is B.
Frequently Asked Questions-FAQs:
Q. 1. What is the difference between electrophilic and nucleophilic substitution reactions?
Answer: A substitution reaction is one in which an atom or group of atoms in an organic molecule is directly replaced by another atom or group of atoms with no change to the remaining part of the molecule. Electrophilic substitution reactions are substitution reactions initiated by electrophiles. Nucleophilic substitution reactions are those involving substitution that begins with a nucleophile attack.
Q. 2. What is Wheland Complex?
Answer: An intermediate carbocation is formed in the second step of the electrophilic aromatic substitution reaction which is known as arenium ion and it is also called as wheland complex or Meisenheimer complex.
Q. 3. Give any three examples of electrophilic aromatic substitution reactions.
Answer: There are many types of electrophilic aromatic substitution but some common types are halogenation of benzene, nitration of benzene, and sulphonation of benzene.
Q. 4. What is the use of an Electrophilic aromatic substitution reaction?
Answer: One of the most important reactions in synthetic organic chemistry is electrophilic aromatic substitution. These reactions are used to produce important intermediates that can be used as precursors in the production of pharmaceutical, agrochemical, and industrial products.