-
Call Now
1800-102-2727
Anti-Aromatic Compounds: Aromatic and Anti-Aromatic compounds, Criteria for Anti-Aromaticity, Examples, Practice Problems & Frequently Asked Questions
In every school, students are separated and taught based on any one of the following three courses they are interested in and capable of-
(i) Engineering course subjects of maths, physics and chemistry
(ii) Medical course subjects of Physics, chemistry and biology and
(iii) Commerce and language subjects
Similarly, in organic chemistry, we have a set of cyclic compounds that are classified as Aromatic, Non-aromatic and Anti-aromatics.
You shall learn about aromaticity and anti-aromatic compounds in this description.
Table of Contents
- Aromatic and Anti-Aromatic Compounds
- Criteria for Anti-Aromaticity
- Examples of Anti-Aromatic Compounds
- Practice Problems
- Frequently Asked Questions(FAQs)
Aromatic and Anti-Aromatic Compounds:
Organic compounds are broadly classified into linear (Aliphatic) and aromatic compounds. These aromatic compounds are either homocyclic or heterocyclic in nature. Whatever it may be, they exhibit some common features so as to be classified as Aromatic compounds.
The common features of these aromatic compounds are-
- They are cyclic compounds
- Have cyclic and conjugated pi-electrons
- Possess, Huckel’s number of pi-electrons = 4n +2, where ‘n’ is any whole number from zero.
- Molecule has a planar structure ie ring-forming atoms should have sp2 hybridization.
There are also some homocyclic and heterocyclic compounds which are similar but lack some of the above-said features of aromatic compounds and hence do not show the special aromatic characteristic of aromatic compounds. A cyclic and planar compound having (4n) instead of 4n + 2 pi-electrons in cyclic conjugation, does not show aromaticity and such organic compounds are referred to as Antiaromatic.
Cyclic compounds not having a conjugate cyclic pi-electron are called non-aromatic compounds.
Criteria for Antiaromaticity:
Let's look at the conditions that a compound needs to meet in order to show antiaromaticity.
The four guidelines that follow list the criteria that define if a substance is aromatic.
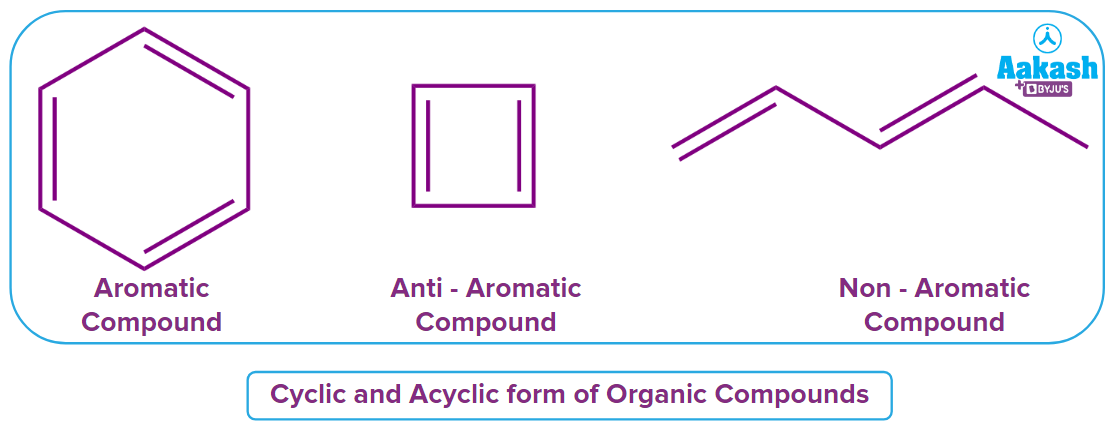
Rule 1: The molecule must have a cyclic structure. The third structure is acyclic and hence non-aromatic
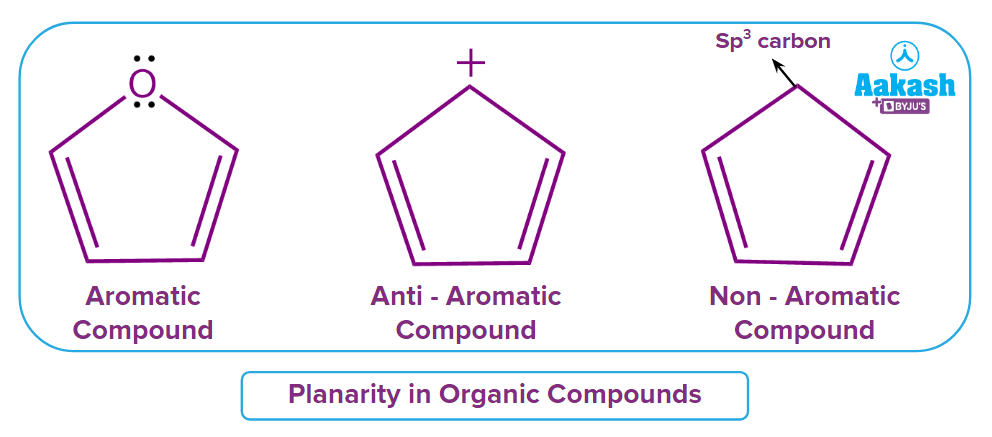
Rule 2: The molecule must be planar. Molecules with sp2 hybridized carbons force planarity in structure. In the first structure four carbons are sp2 hybridzed and overlap with pi-orbital electrons of oxygen hence will be a planar molecule. In the second structure, all the carbons atoms are of sp2 hybridized and will be planar. The third one with terminal sp3 carbon will not be planar.
Rule 3: Within the ring, the molecule must have a fully conjugated -electron system.
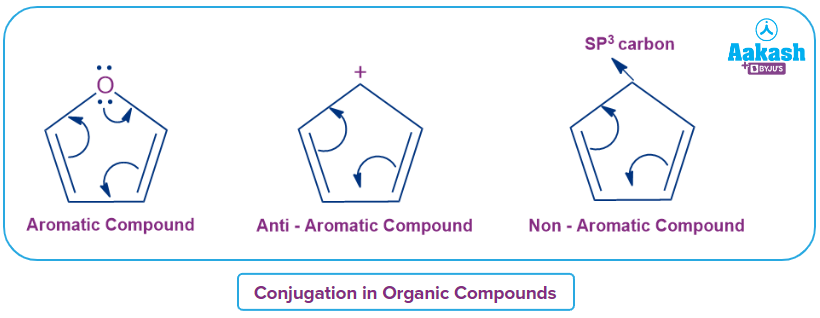
In the first structure, the two double-bond pi-electrons are in conjugation with the pi-electrons of the oxygen atom in the ring. All six electrons in the five-membered ring are conjugated. In the second structure, the two double-bond pi-electrons are in conjugation with the empty orbitals of the carbonium carbon. In the third structure, the sp3 carbon is already saturated and does not take part in any interaction with another pi- electrons of the molecule. Hence there is no complete conjugation in the ring structure.
Rule-4: The molecule must have 4n-electrons, where n can be any integer inside the conjugated -system.
This varies from aromaticity in the fourth criterion: aromatic molecules have (4n+2) electrons in the conjugated system and so follow Hückel's rule. Non-aromatic compounds are either noncyclic, nonplanar or lack a fully conjugated system within the ring.
Examples of Anti aromatic compounds:
1. Cyclobutadiene:
Let's talk about whether cyclopentadiene is an antiaromatic compound or not. It is clear that it is a cyclic molecule with a planar structure. It also has four electrons, which may be expressed as {4(1)=4}, making it an anti-aromatic molecule.
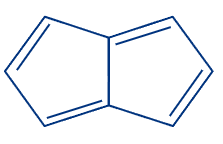
2. Pentalene:
Two fused cyclopentadiene rings make up the polycyclic hydrocarbon known as pentalene. Its molecular structure is C8H6. Because it contains 4n- electrons, where n can be any number and is a cyclic molecule with a planar structure it is considered an antiaromatic compound.
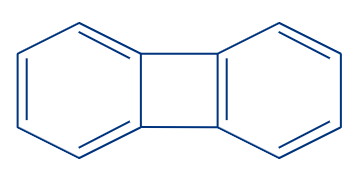
3. Biphenylene:
A polycyclic hydrocarbon called biphenylene is made up of two benzene rings connected by two bridging bonds (instead of a typical ring fusion), resulting in a 6-4-6 arene system. One of the earliest -electronic hydrocarbon systems to exhibit antiaromaticity was the resultant planar structure.
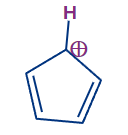
4. Cyclopentadienyl cation:
In the cyclopentadiene ring, one of the carbon is having sp3 hybridisation and it lacks an uninterrupted cyclic pi-electron cloud, it is not an aromatic molecule because. When a hydride anion (H-) is withdrawn from the cyclopentadiene sp3 hybridised ring carbon, the cyclopentadienyl cation is generated, and the hybridization of the sp3 hybridised ring carbon is changed from sp3 to sp2.
The cyclopentadienyl cation that has the carbonium cation in conjugation with the double bond present in the ring. and the sp2 hybridization makes the molecule planar. Thus the cation will be planar and have a cyclic pi-electron cloud and it does contain 4n electrons (n is equal to 1 as there are 4 pi electrons). It is hence antiaromatic.
Practice Problems:
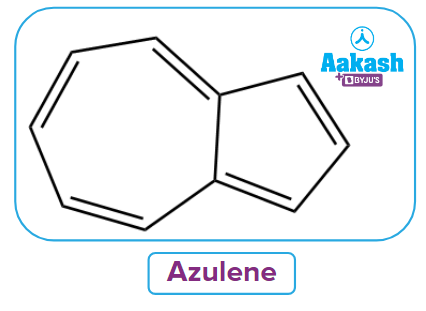
Q1. Azulene is an example of
(A) an Aromatic benzenoid compound
(B) an Aromatic non-benzenoid compound
(C) an Aromatic heterocyclic compound
(D) None of the above
Answer: (B)
Solution: The term "non-benzenoid aromatic systems" refers to aromatic compounds without six-membered benzene ring. It has two non-benzene fused rings. Azulenes are aromatic non-benzenoid molecules that have been fused. These molecules can be found in anti-blemish cosmetic products.
Q2. Anti-aromatic compounds are
(A) Highly unstable
(B) Highly Stable
(C) Unstable
(D) None of the above
Answer: (A)
Solution: The stability of the compound will increase as the delocalization energy (resonance energy) increases. The delocalization energy of antiaromatic compounds is zero, making them extremely unstable, whereas the delocalization energy of aromatic compounds is not zero, making them quite stable.
Q3. Pyridine is an example of
(A) An Aromatic benzenoid compound
(B) Aromatic non-benzenoid compound
(C) An Aromatic heterocyclic compound
(D) None of the above
Answer: (C)
Solution: Aromatic heterocyclic compounds are those with sigma bonds and delocalized electrons between carbon atoms and another element in the ring system, such as oxygen, nitrogen, phosphorus, or sulphur. The ring system in this system has more than one sort of atom.
Q4. Among the following, which is an antiaromatic compound?
(A) Borole
(B) Benzene
(C) Toluene
(D) Furan
Answer: (A)
Solution: Boroles belong to the metallole category of molecules, which are heterocyclic 5-membered rings containing boron in the ring. As such, they can be thought of as structural analogues of cyclopentadiene, pyrrole, or furan, with boron substituting a carbon, nitrogen, or oxygen atom. Although Hückel's rule cannot be applied literally to borole, it is considered antiaromatic due to its four electrons. As a result, boroles have distinct electrical properties that are not present in other metalloles.
Frequently Asked Questions(FAQs):
Q1. Define homoaromaticity?
Answer: Typically, aromatic molecules have sp2 hybridised carbon atoms. As a result, they have continuous p-orbital overlap and a triangular planar geometry. However, one sp3 hybridised carbon atom exists in homoaromatic compounds, disrupting the continuous overlap of p-orbitals. The behaviour of these compounds is aromatic despite the discontinuity in the p-orbital overlap. In other words, by avoiding that one sp3 atoms, these molecules create a stabilised cyclic conjugated system.
Q2. What exactly are quasi-aromatic compounds?
Answer: These are the types of aromatic compounds that participate in conjugation to exhibit aromatic behaviour and have a charge on the ring. A quasi-aromatic compound, to explain it simply, is an ionic compound with a counter ion that participates in the molecule's (4n+2) electron system.
The cyclopropenium ion and cyclopentadienyl ion are examples of quasi-aromatic compounds.
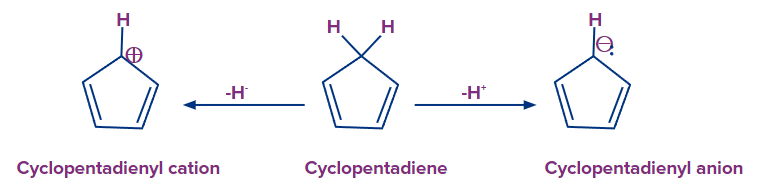
Q3. The cyclopentadienyl cation is antiaromatic, whereas the anion is aromatic. Justify?
Answer: Cyclopentadienyl cation and anion are generated from cyclopentadiene through the removal of a hydride ion and the abstraction of a proton, respectively. Because of the existence of an sp3 hybridised ring carbon on its ring, cyclopentadiene is not an aromatic molecule because it lacks an uninterrupted cyclic pi-electron cloud. When a hydride anion (H-) is withdrawn from the cyclopentadiene sp3 hybridised ring carbon, the cyclopentadienyl cation is generated, and the hybridization of the sp3 hybridised ring carbon is changed from sp3 to sp2.
The cyclopentadienyl cation that is created as a result of this conversion of hybridization will be planar and have a cyclic pi-electron cloud. However, because it lacks (4n+2) electrons, it cannot be aromatic according to Huckel's rule of aromaticity. However, it does contain 4n electrons (n is equal to 1 as there are 4 pi electrons). It is hence antiaromatic.
When a proton is extracted from the cyclopentadiene sp3 hybridised ring carbon, the cyclopentadienyl anion is generated, and the hybridization of the sp3 hybridised ring carbon is changed from sp3 to sp2. As a consequence of this hybridization transformation, the cyclopentadienyl anion generated will be planar with a cyclic pi-electron cloud. Furthermore, it fits Huckel's rule for aromaticity because it has (4n+2) electrons (n equals 1 because there are 6 pi electrons) and so is aromatic. Cyclopentadienyl anion is thus an aromatic molecule.
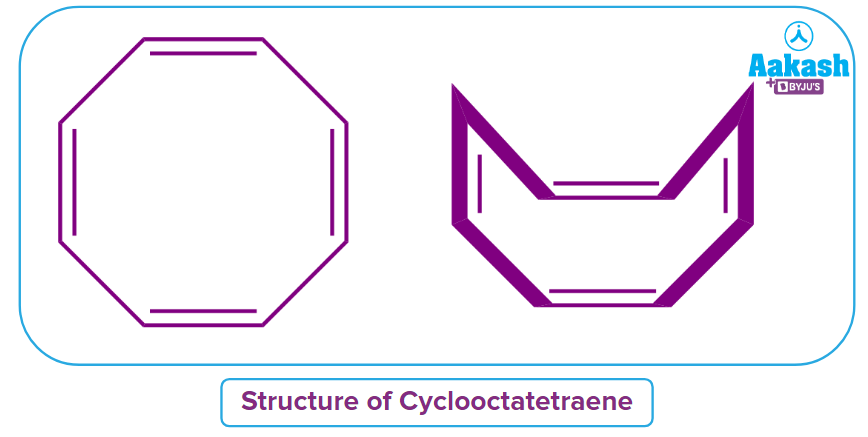
Q4. Why is cyclooctatetraene a non-aromatic compound?
Answer: The provided compound, cyclooctatetraene, looks to be antiaromatic, but it is actually non-aromatic. Because of the significant steric hindrance, the compound loses its planarity and appears as a tub, as illustrated in the image, and is also known as a tub-shaped structure.