-
Call Now
1800-102-2727
Ammonia: Chemical Formula, Nature, Structure, Preparation, Physical and Chemical Properties
Ammonia (NH3) - Structure, Preparation, Physical and Chemical properties, Uses, Analytical Tests, Practice Problems and FAQ
Beautifully coloured hair and flaunting it in front of the camera is a current trend among millennials. We've all seen advertisements with women flaunting themselves, the spotlight shining on their gorgeously coloured hair. There are almost an infinite number of hair colour brands out there, all vying for the attention of all potential customers by using vibrant hair colours or even taglines like "No Ammonia" to justify their product as safe and guilt-free to use.

Did you know that ammonia is an integral part of making hair dyes?
Making a hair dye "ammonia-free" makes it a little safer for your hair, but it also makes the colour semi-permanent or less durable. But, yes! in some cases, simply highlighting the name of a chemical is enough to raise the brand pricing for a specific product.
Wouldn't you like to learn more about and comprehend the chemistry of this chemical? Let us delve deeper to learn about the one who never ceases to amaze - "Ammonia"!
TABLE OF CONTENTS
- Nature
- Hybridisation and Structure
- Preparation
- Haber Process - Large Scale Production of Ammonia
- Physical Properties
- Chemical Properties
- Uses
- Analytical Tests for Ammonia
- Practice Problems
- Frequently Asked Questions - FAQ
Nature
Ammonia (NH3) is a colourless inorganic compound with a pungent odour which is similar to that of urine. Ammonia is present in small quantities in air the air and soil. It is formed by the decay of nitrogenous organic matter.
Ammonia (NH3) is an inorganic compound that is colourless and has a strong, unpleasant smell akin to that of urine. Both the air and soil contain trace amounts of ammonia. It is created when nitrogenous organic matter decays.
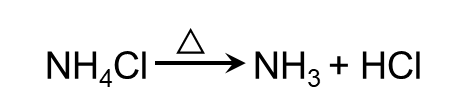
Ammonia takes its name from the worshippers of the Egyptian god Amun - the Ammonians because they used ammonium chloride (NH4Cl), also called sal volatile, in their rites. Ammonium chloride’s natural source is near the cracks of volcanoes. It is generally very heated up here, and hence gets decomposed to produce pungent-smelling ammonia gas. The IUPAC name of ammonia is azane. It is alkaline in nature.
Hybridisation and Structure
Ammonia is made of nitrogen and hydrogen. The atomic number of nitrogen is 7 and its electronic configuration is 1s2 2s2 2p3.
In the process of ammonia formation, one 2s orbital and three 2p orbitals of nitrogen combine to form four hybridised orbitals having equal energy and hence it is said to be a sp3 hybridised molecule. Three half-filled sp3 orbitals of nitrogen form bonds with three hydrogen atoms. However, the fourth sp3 orbital is a non-bonding hybridised orbital and contains a lone pair. This lone pair is the main reason for the basicity of ammonia.
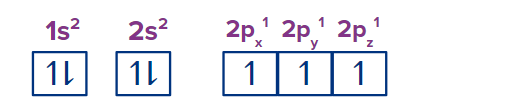
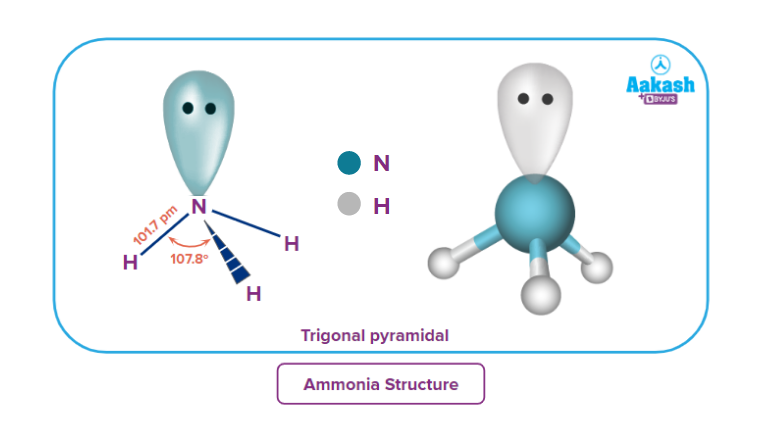
Due to the sp3 hybridisation and presence of a lone pair, ammonia is trigonal pyramidal in shape.
Preparation
- For research or small scale small-scale production, ammonia gas can be produced by slowly heating ammonium chloride NH4Cl and calcium hydroxide (Ca(OH)2) solutions.
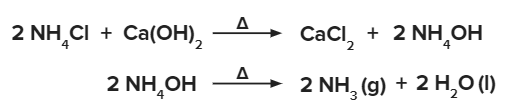
Since ammonia is quite soluble in water, it can not be gathered over water. Quicklime is used to dry ammonia gas.
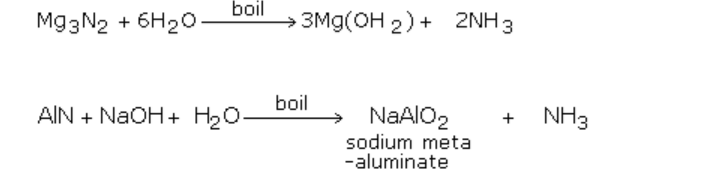
- Nitrides of metals like aluminium and magnesium undergo hydrolysis with water or alkali to produce ammonia.
- Ammonia can be produced from urea in the following manner.
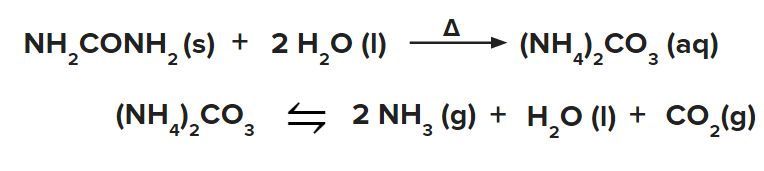
Haber Process-Large Scale Production of Ammonia
Haber process is used for the large-scale production of ammonia. The main reaction involved is:
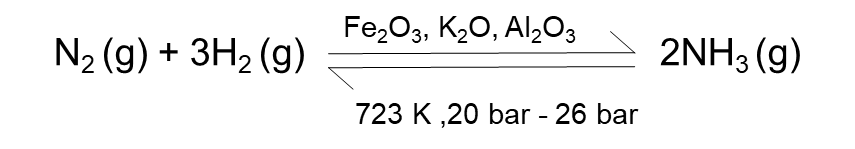
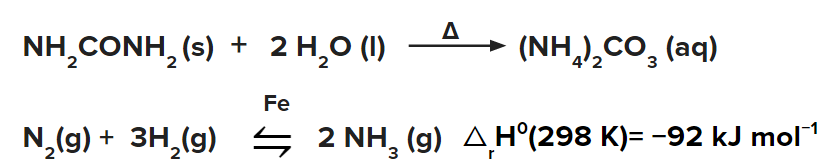
According to Le Chatelier’s principle, “ high pressure would favour the formation of ammonia as the product side has a lesser number of moles.”
Optimum conditions needed: Pressure- (20 bar - 26 bar) and temperature 723 K
The activation barrier for the dissociation of N2 and H2 in the gas phase is very high.
Without a catalyst, the reaction between N2 and H2 occurs slowly. Iron oxide with a small amount of K2O and Al2O3. Earlier, iron was used as a catalyst with molybdenum (Mo) as a promoter.
- The impurities from nitrogen and hydrogen gases are removed by a process called scrubbing.
- After scrubbing, the gases are mixed together and it is then passed through a compressor. The mixture is then compressed at a pressure of 200 atm.
- The compressed gases are then passed through a converter, where the gases are heated at a temperature of 723 K and a pressure of 200 atm. The nitrogen reacts with hydrogen to form ammonia, however, only approximately 15% of the gas is formed.
- The mixture of ammonia, hydrogen, and nitrogen is removed from the converter. It is cooled where it liquefies in the tank and hence collected.
Physical Properties
- Ammonia is a colourless, pungent gas.
- The boiling point of ammonia is about −33.35 °C and is said to freeze at about −77.7 °C.
- The density of ammonia is 0.73 kg m-3.
- Ammonia has a high heat of its vapourisation (1370 J g-1) and hence, it is handled as a liquid in the form of thermally insulated containers.
- In the solid and liquid states, it is associated via hydrogen bonds.
- Ammonia molecule is polar due to strong intermolecular hydrogen bonding.
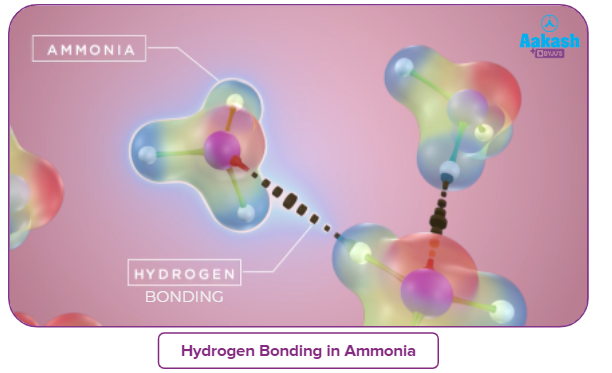
- It has a dielectric constant of 22 at −34 °C (−29 °F) which is lower than that of water. Hence, it serves better as a solvent for all the organic materials.
- The aqueous solution of ammonia is basic as it can produce hydroxide ions ( and OH-).
- The molar mass of ammonia ia 17.031 g mol-1.
Chemical Properties
- Ammonia has self-ionising property, although it is less than that of water.
- Ammonia, which is a hydride of the element nitrogen of the pcintogen pnictogen family, is thermally highly stable.
- Ammonia acts as a base. It has a lone pair of electron electrons which can be donated. Hence, it is a Lewis base.
- It forms ammonium salts with acids.
- As a weak base, it precipitates the hydroxides of many metals from their salt solutions.
This is a double decomposition reaction.
- Ammonia acts as a Lewis base due to the presence of a lone pair on nitrogen and hence, it acts as a neutral ligand forming coordination complexes with metal ions. It donates the electron pair and forms a linkage with metal ions. The formation of such complex compounds finds applications in the detection of metal ions.
[Deep blue solution]
[Colourless solution formed]
[White ppt of silver chloride turns colourless on passing ammonia]
- Ammonium salts decompose readily on heating. If the anion is not oxidising, then ammonia is released. If anion is oxidising, then is oxidised to N2 or N2O.

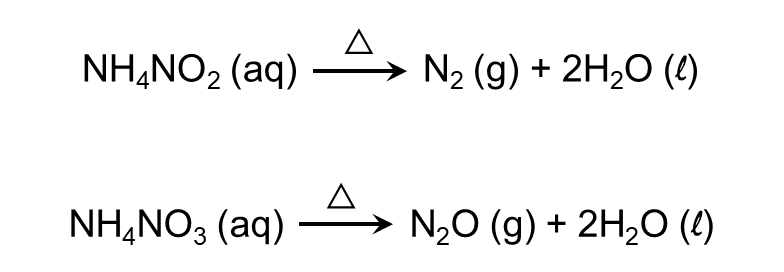
- Ammonia is combustible in nature.
Uses
- Ammonia is used to produce nitrogenous fertilisers like ammonium nitrate, ammonium phosphate, ammonium sulphate, urea, etc.
- Liquid ammonia is used as a refrigerant.
- Ammonia is one of the main ingredients in multiple household cleaning products. It is used as a cleaning agent and can be used to remove stains or clean mirrors, tubs, sinks, windows and more.
- Ammonia is useful in several industrial processes like in manufacturing nitric acid by Ostwald’s process and in manufacturing sodium carbonate by Solvay’s process.
- In the petroleum industry ammonia is used to counterbalance the acid constituents of oil which are in crude form. It also helps to keep equipment free from corrosion.
- Ammonia is used in the mining industry for the extraction of various metals.
- Dissociated ammonia is used in operations like carbonitriding, nitriding, furnace brazing, bright annealing, sintering, atomic hydrogen welding and other operations.
- Hydrazine made from the reduction of ammonia with hydrogen has great potential to serve as rocket fuel.
Analytical Test for Ammonia
- The ammoniacal odour of the compound is effectively perceivable, having a trademark pungent smell.
- It turns wet red litmus blue and moist turmeric paper brown in colour.
- A glass bar dunked in concentrated HCl when conveyed near ammonia causes thick white exhaust.
- When added to a solution of copper sulphate, ammonia turns the solution deep blue.
- When treated with Nessler’s reagent (basic arrangement of K2[HgI4]), ammonia gives a precipitate which is brown in colour
Practice Problem
Q1. On passing ammonia through a solution of copper sulphate, what observation is made?
A. Evolution of a gas
B. Bronze-coloured solution obtained
C. Deep blue solution obtained
D. Deep blue precipitate
Answer: When ammonia is passed through a solution of copper sulphate, a deep blue solution of an ammoniated complex of copper ion (Tetraammine copper (II) ) is obtained. Ammonia donates the electron pair and forms linkage a linkage with metal ions.
[Deep blue solution]
So, option C) is the correct answer.
Q2. What happens when ammonium sulphate reacts with sodium hydroxide?
Answer: On reacting with ammonium sulphate, sodium hydroxide leads to the evolution of ammonia gas along with the formation of sodium sulphate.
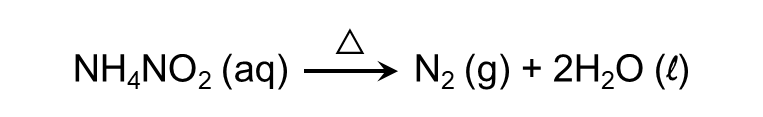
Q3. The product obtained on heating ammonium nitrite will be:
A. Nitrous oxide
B. Nitrogen trioxide
C. Nitrogen dioxide
D. Nitrogen
Answer: Ammonium salts decompose on heating. Ammonium nitrite produces nitrogen gas on heating.
Q4. Ammonia is a/an:
A. Anionic ligand
B. Lewis Acid
C. Lewis Base
D. Electrophile
Answer: Ammonia has a lone pair of electrons on the nitrogen atom. It acts as a Lewis base as it can donate the electron pair. Since it is a neutral molecule, it is a neutral ligand. So, option C) is the correct answer.
Frequently Asked Questions - FAQ
Q1. What are the impacts of ammonia on health?
Answer: Ammonia can be present in the air at a level of only about 50-60 ppm, and at levels of 100-200 ppm, it sharply irritates the eyes and lungs. At even higher concentrations, it makes the lungs fill with fluid and can quickly cause death.
Q2. Why is the iron catalyst used for the Haber process?
Answer: The Haber process employs an iron catalyst because iron is long-lasting, inexpensive, and effective at catalysis. A surface for the reaction to occur is provided by finely divided solid Fe. Nitrogen and hydrogen gases adsorb on the catalyst surface, a reaction takes place, and the products diffuse out.
Q3. How is hydrogen gas obtained for the Haber process?
Answer: Methane from natural gas is the main source of hydrogen. In a high-temperature and pressure pipe inside a reformer with a nickel catalyst, steam reforming is carried out, separating the carbon and hydrogen atoms in the natural gas.
Q4. What factors affect the Haber process?
Answer: Since it is a reversible process, in order to maximise ammonia yield, we need to follow optimum reaction conditions in accordance with Le Chatelier’s principle. Optimum conditions needed are: Pressure- (20 bar - 26 bar) and temperature 723 K.
Q5. Why is the Haber process important?
Answer: The Haber process is still necessary because it produces ammonia, which is vital for fertilisers and many other purposes. Every year, the Haber cycle produces around 500 million tons of fertilisers (453 billion kilograms). This fertiliser helps feed about 40% of the population of the world.
Q6. Why is ammonia used in hair colour?
Answer: Ammonia, being alkaline in nature raises the pH level of the hair during the colouring process. Then, it leads to the lifting up of the cuticles of the hair fibre and allows the colour to be deposited onto the inner part of the hair protected by the cuticles. Ammonia also lightens the hair’s natural pigment so it can be re-coloured.


