-
Call Now
1800-102-2727
Alkyl Halides: Classifications, Preparations, Physical and Chemical Properties, Practice Problems and Frequently Asked Questions
These kinds of chemicals have several applications in both business and everyday life.
They are utilised as solvents for nonpolar molecules and as starting materials in the synthesis of a wide variety of chemical compounds.
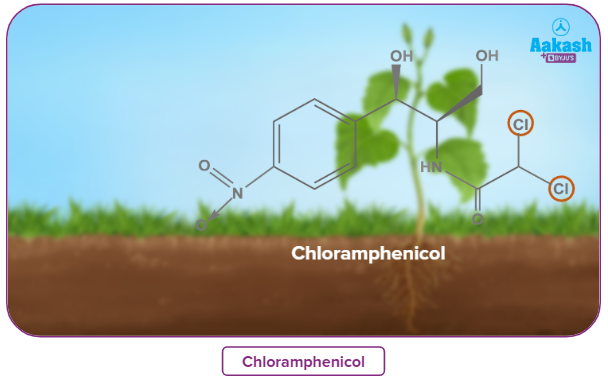
Chloramphenicol, a chlorine-containing antibiotic generated by soil bacteria, is particularly successful in the treatment of typhoid fever.
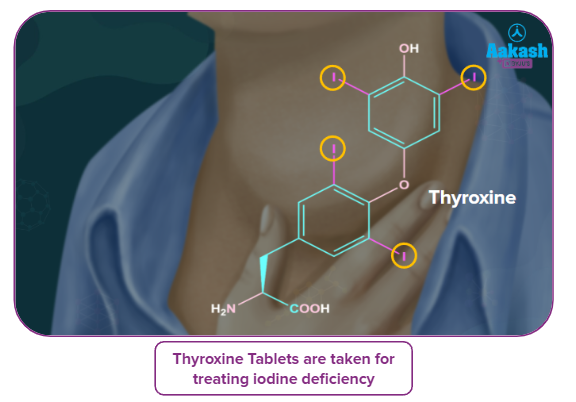
Our bodies manufacture the iodine-containing hormone thyroxine, a lack of which causes the condition goitre.
Synthetic halogen compounds, such as chloroquine, are used to treat malaria, while halothane is used as an anaesthetic during surgery. Certain completely fluorinated substances are being investigated as potential blood substitutes in surgery.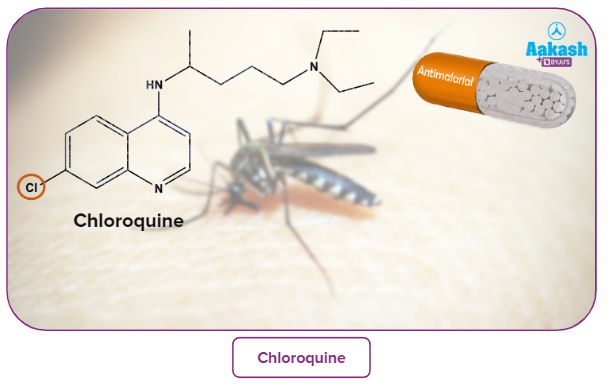
Table of content:
- Introduction
- Classification of alkyl halides
- Preparations of alkyl halides
- Physical properties of alkyl halides
- Chemical properties of alkyl halides
- Practice problems
- Frequently asked questions
Introduction
Compounds derived from alkanes, most of which contain one or more halogens, are alkyl halides, also known as haloalkanes. Haloalkanes can also be assumed to be a subgroup of common classes of halocarbons.
Alkyl halides or haloalkanes are formed by replacing the hydrogen atoms of aliphatic hydrocarbons with halogen atoms (fluorine, chlorine, bromine, or iodine). Any organic precursors such as alkanes, alkenes or alcohols and carboxylic acids can also be derived from them. Halogenated alkyl usually contains a hydrogen atom attached to the alkyl group of the sp3 hybridised carbon atom. In general, the formula for alkyl halides is RX.
Classification of alkyl halides:
Based on the number of halogen atoms:
The classification depends primarily on whether the structure contains one, two, or more halogen atoms. The categories include:
1. Mono Haloalkane : Monohaloalkanes are the alkanes having one halogen atom
Example: [where X can be Cl,F,Br or I]
2. Dihaloalkane: Dihaloalkanes are the alkanes having Two halogen atom
Example: [where X can be Cl,F,Br or I]
3. Tri haloalkane: Trihaloalkanes are the alkanes having three halogen atom
Example: [where X can be Cl,F,Br or I]
Based on the Position of the Halogen atom Along the Chain of Carbon Atom:
Primary alkyl halide:
When a carbon atom which is attached to only one alkyl group and attached to a halogen atom, it is known as a primary haloalkane (1o haloalkane). The complex structure of attached alkyl groups does not play any role in the degree of the alkyl halides because there is only one linkage to an alkyl group from the carbon which holds the halogen. Examples of primary alkyl halides include:
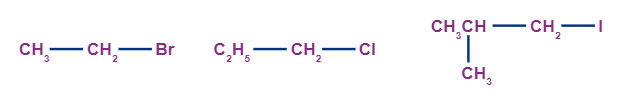
Secondary alkyl halides:
When a carbon atom which is attached to two other alkyl groups (which may be the same or different) and attached to a halogen atom, is known as a secondary haloalkane (2o haloalkane). Examples of secondary alkyl halides are:
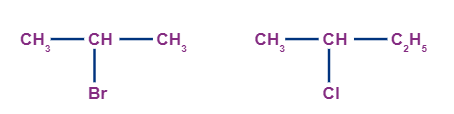
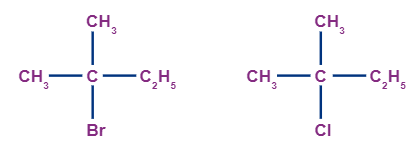
Tertiary alkyl halides:
When the carbon atom which is attached to three other alkyl groups (which may include any combination of the same or different) is bonded to the halogen atom, it is known as tertiary haloalkane (3° haloalkane). Examples of tertiary alkyl halides include:
Preparations of alkyl halides:
1. Reaction of Alcohol with Halides of Phosphorus (PX3 or PX5) :
This reaction helps in the formation of chloroalkanes, bromoalkanes and iodoalkanes. In this reaction, phosphorus halide exchanges the alcohol functional group (-OH) with the corresponding halide. The reaction proceeds as follows:
2. Reaction of Alcohol using thionyl chloride:
Of the three alcohol reactions, this reagent is the preferred and most convenient. Alcohol reacts with thionyl chloride (SOCl2) to form an alkyl chloride. However, the by-products formed by this reaction are gaseous. But by-products can also easily release alkyl halides to the atmosphere in their purest form. This technique supports the processing of pure alkyl halides.
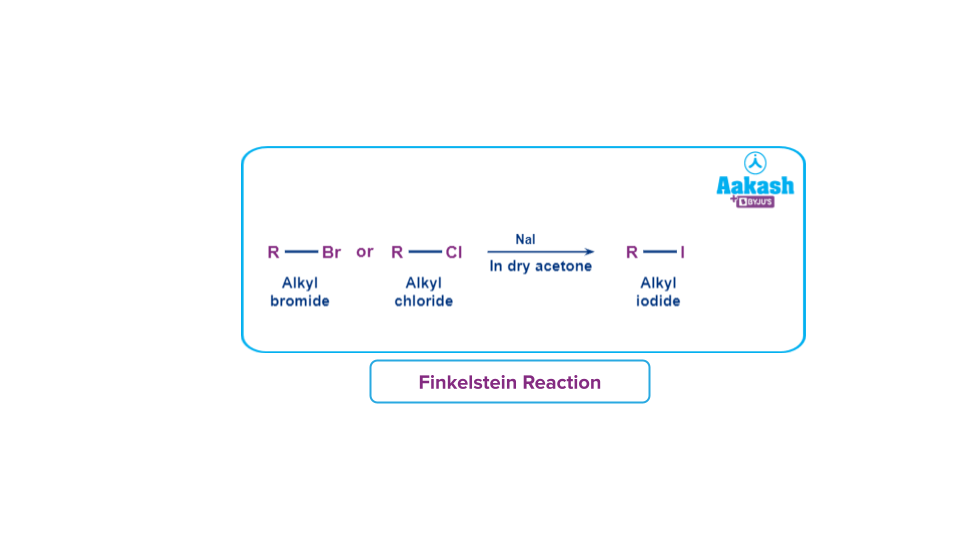
3. Finkelstein reaction:
The Finkelstein reaction, named after the German chemist Hans Finkelstein is an SN2 reaction (Bimolecular Nucleophilic Substitution reaction) in which one halogen atom is exchanged for another.
The classic Finkelstein reaction involves converting an alkyl chloride or alkyl bromide to an alkyl iodide by treating it with an acetone solution of sodium iodide. Sodium iodide dissolves in acetone but not sodium chloride or sodium bromide.
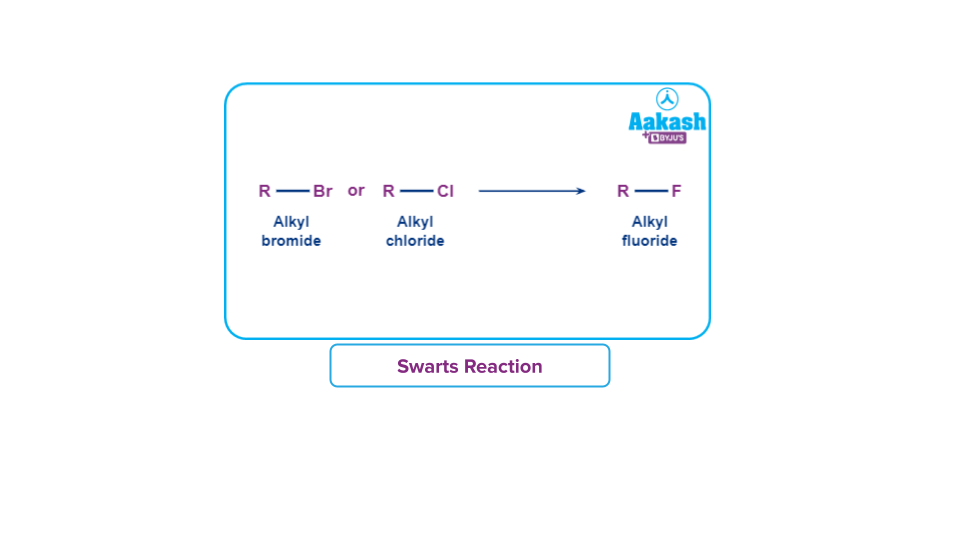
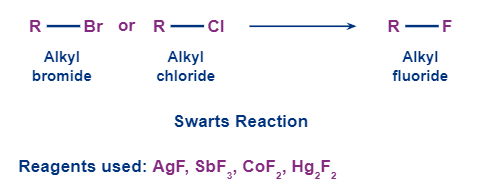
4. Swarts reaction:
Swarts’ reaction is generally used to get alkyl fluorides from alkyl chlorides or alkyl bromides. This is done by heating of the alkyl chloride/bromide in the presence of the fluoride of some heavy metals (silver fluoride or mercurous fluoride for example).
Physical properties of alkyl halides:
Alkyl halides are colourless in their pure form. Bromides and iodides, on the other hand, produce colour when exposed to light. The production of colour is explained by the decomposition of halogens in the presence of light. Many volatile-nature halogen compounds have a pleasant odour.
Odour: Alkyl Halides have a pleasant odour in their pure state, whereas all higher alkyl halides have no odour.
Colour: Alkyl Halides are colourless in their pure form. Iodoalkanes and bromoalkanes, on the other hand, develop colour when exposed to light after being stored for a long time.
Melting Point: The strength of a compound's lattice structure determines its melting point. The melting points of isomeric di halobenzene varies despite the fact that their boiling points are nearly identical. Because the crystal lattice becomes more densely packed with the molecules. As a result, more energy is required to split the lattice structure, raising the compound's melting point.
Boiling Points: Because alkyl halides have polarity and strong dipole-dipole attraction between their molecules, as well as a greater magnitude of van der Waals forces, their boiling points are high.
- The boiling point of the same alkyl group of the halogens is of the order RCl<RBr<RIThis is so because as there is an increase in the size of the halogen atom, the magnitude of the van der Waals force will increase.
- The boiling point of isomeric haloalkanes decreases as branching increases because the surface area of the molecule decreases, causing intermolecular forces of attraction to diminish and hence the boiling point to decrease. The order is .
- If there are identical halogens, the boiling point depends on the molecular mass and increases with increasing molecular mass as the size of the alkyl group increases, the magnitude of the van der Waals force increases. The order of boiling point is: .
- If the number of halogen atoms increases, the boiling point of the compound also increases because of the increased van der Waals forces.
Density: Density is proportional to the mass of any compound. Therefore, density increases as mass increases in the homologous series. Therefore, fluorinated derivatives have smaller density than chlorinated derivatives, and chlorinated derivatives have a smaller density than brominated derivatives. Alkyl bromide and alkyl iodide are heavier than water while alkyl chloride is lighter. The order of density is: RI>RBr>RCl.
Solubility: In water, alkyl halides are very slightly soluble. To dissolve a compound and break the attraction between a halogen and a carbon atom, a relatively larger amount of energy is required. The alkyl halides are polar in nature because they do not form hydrogen bonds with water molecules and are therefore poorly soluble in water. Solvents in which they can dissolve are organic solvents such as ether, alcohol, and benzene.
Chemical properties of alkyl halides:
Haloalkane chemical reactions are classified into three types:
- Nucleophilic substitution reaction
- Elimination reaction
- Reaction with metals
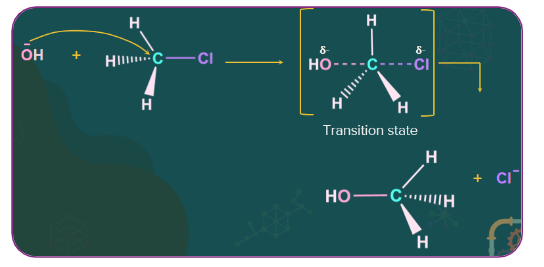
Nucleophilic substitution reaction:
In this type of reaction, a nucleophile reacts with a haloalkane that has a partial positive charge on the carbon atom bonded to the halogen. The substitution reaction occurs and a halogen atom called the leaving group leaves as a halide ion. Since the substitution reaction is initiated by a nucleophile, it is called a nucleophilic substitution reaction.
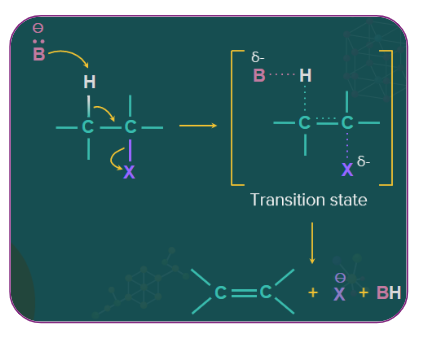
Elimination reaction:
When a haloalkane with a hydrogen atom is heated with an alcoholic solution of potassium hydroxide, it results in the removal of a hydrogen atom from the β-carbon atom and a halogen atom from the α-carbon atom.As a result, one of the products is an alkene. Since the β-hydrogen atom participates in the elimination, it is often referred to as the β-elimination reaction.
Reaction with metals:
Most organic chlorides, bromides, and iodides react with some metals to form compounds containing metal-carbon bonds. Such compounds are called organometallic compounds. The product is formed by the reaction of the alkyl/aryl halide with metallic magnesium in dry ether.
[X=Cl,Br and I]
Meanwhile, the Grignard reagent is formed in this reaction which can react with any source of protons leading to the formation of hydrocarbons.
Practice problems:
Q.1. Why are alkyl halides considered to be very reactive compounds with nucleophiles?
(A) they have an electrophilic carbon and a leaving group
(B) they have a good leaving group and have an nucleophilic carbon
(C) they have an electrophilic carbon
(D) they have good electrophilic carbon and good leaving groups
Answer: (D)
Solution: Because they have an electrophilic carbon and a good leaving group, alkyl halides are considered to be very reactive towards nucleophiles. As we move down the periodic table, larger halides will be able to distribute their charge over a larger volume, making them less reactive (less basic). As a result, fluoride has a much lower leaving group tendency than the other halides. Alkyl halides are therefore useful in nucleophilic substitution reactions.
Q.2. Ethyl chloride on reaction with hot aqueous solution of silver oxide gives
(A) Ethyl alcohol (B) Methyl alcohol
(C) dimethyl ether (D) None of the above
Answer: (A)
Solution: Ethyl chloride on reaction with hot aqueous solution of silver oxide gives ethyl alcohol. The reaction is as follows:
Q.3. Which alkyl halide has the highest boiling point?
(A) 1-chloro pentane (B) 1-bromo pentane
(C) 1-iodo pentane (D) All have same boiling point
Answer: (C)
Solution: The boiling point of the halogens having same alkyl group is of the order RCl<RBr<RI. This is so because as there is an increase in the size of the halogen atom, the magnitude of the van der Waals force will increase.
Q.4. Which C-X bond has the highest bond energy per mole?
(A) C-Br (B) C-Cl
(C) C-F (D) C-I
Answer: (C)
Solution: Bonding energy depends on many factors: electron affinity, the size of the atoms participating in the bond, the difference in electronegativity and the overall structure of the molecule. There is a general trend that the shorter the bond length, the higher the bond energy. That's why C-F will have the highest bond energy than other C-X bonds.
Frequently asked questions:
Q1. Why are alkyl halides polar?
Answer: The alkyl halides are polar in nature due to the difference in electronegativity between the carbon atom and the halogen atom. Halogens are more electronegative than carbon so the bonding electrons move towards the halogen atom making the bond polar.
Q2. Explain why alkyl halide molecules though polar are immiscible in water?
Answer: Alkyl halides are polar molecules, therefore, their molecules are held together by dipole-dipole attractions. The molecules of water are held together by hydrogen bonds,since the new force of attraction between water and alkyl halide molecules are weaker than the forces of attraction already existing between alkyl halide-alkyl halide molecules and water-water molecules, therefore, alkyl halides are not soluble in water. Alkyl halides are neither able to form hydrogen bonds nor able to break the hydrogen bonding network of water molecules. Hence, they are almost immiscible in water.
Q3. Why reaction of alcohol with thionyl chloride is the best method for the preparation of alkyl halides?
Answer: The best method of preparation of alkyl halides is a reaction of alcohol with SOCl2/ Pyridine because by-products formed in the reaction are SO2 and HCl which are gaseous and evaporate into the environment, leaving pure alkyl chlorides behind
Q4. What is the difference between elimination and substitution reaction?
Answer: The difference between substitution and elimination reactions is that substitution responses one replacement with another while elimination reactions simply remove the substituents from the parent molecule.


