-
Call Now
1800-102-2727
Addition of Halogen Acid to Alkynes- Addition Reaction, Mechanism, Addition of Hydrogen Halide, Practice Problems and FAQs
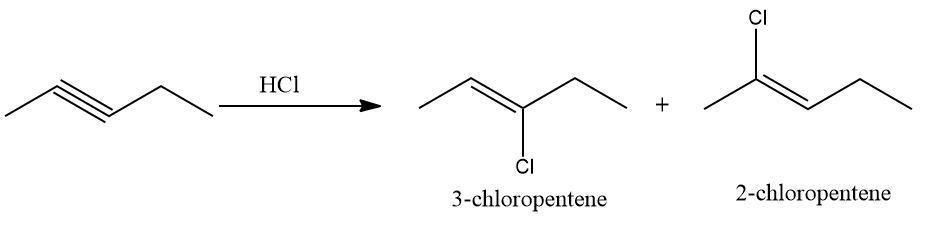
Do you know what the expected product is when 1 mole of hydrogen chloride reacts with Pent-2-yne? Will it be 2-chloro pent-2-ene or 3-chloro pent-2-ene?
Confused?
So, by understanding the addition of hydrogen halides in alkyne in detail and its mechanism, this confusion can be excluded. I assure you that by the end of this discussion, you will be able to easily answer these types of difficult questions.
Table of Contents
- Addition Reactions for Alkynes
- Mechanism
- Addition of hydrogen halides to alkynes
- Practice Problems
- Frequently Asked Questions
Addition Reactions for Alkynes
Alkyne chemistry refers to the chemistry associated with carbon-carbon triple bonds. Because of the presence of loosely held pi-electrons, alkynes undergo electrophilic addition reactions. Because alkynes contain a triple bond, halogens, water, and other substances can be added to them via the addition reaction.
Mechanism
Let us consider the addition of any compound (HZ) to an alkyne.
Step 1: Addition of electrophile
When we add one equivalent of an electrophile (let’s say H+) to an alkyne, it forms a vinyl carbocation. This is an electrophilic addition reaction. When a protic acid (HX) is added to an asymmetric alkene, the π electrons shift in such a way that the positive charge is present on the carbon attached to the alkyl group so that the carbocation is stabilized by the +I effect of the alkyl group and also by the hyperconjugation of respective ⍺-hydrogen atoms.
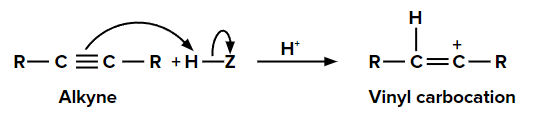
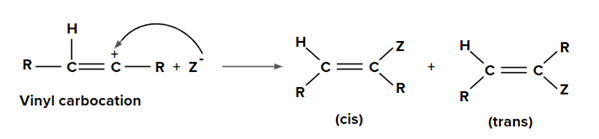
Step 2: Addition of nucleophile (Z-) to vinyl carbocation can give both cis and trans products but in general trans addition is favoured over syn addition.
Addition of hydrogen halides to alkynes
When two molecules of HX (HCl, HBr, or HI) are added to the alkynes, geminal dihalides (in which two halogen atoms are attached to the same carbon atom) is formed.
According to the Markovnikov rule, the addition of HX to unsymmetrical alkynes takes place.
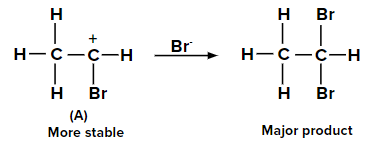
Adding HBr to ethyne according to the Markovnikov rule
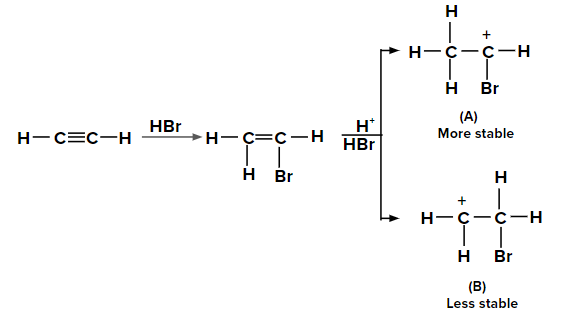
When the second molecule of HBr is added to bromoethene, two possible carbocations can be
formed. The carbocation labeled as (A) is more stable than (B) due to the +M effect of the -Br
group and also by the hyperconjugation of three ⍺-hydrogen atoms. Hence, the major product is
obtained from carbocation (A).
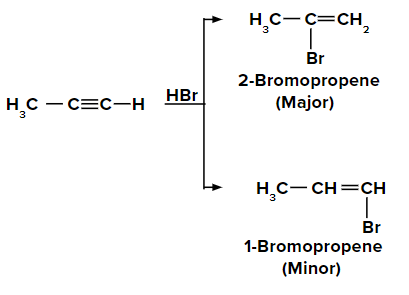
Adding HBr to terminal alkyne like propyne according to the Markovnikov rule
On adding the first molecule of HBr to propyne, we obtain 2-bromopropene and 1-bromopropene as the major and minor products, respectively due to the Markovnikov rule.
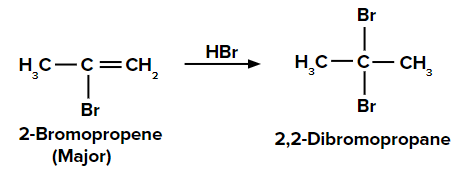
When the second molecule of HBr is added to 2-bromopropene, we get the final product as 2,2-dibromopropane.
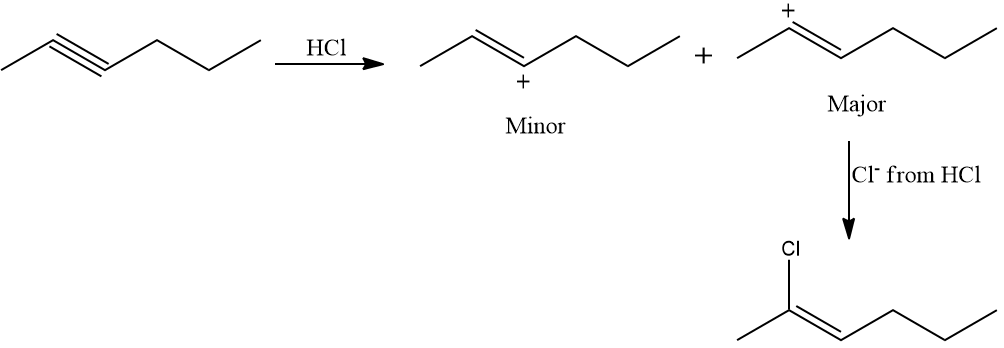
Addition of HCl to internal unsymmetrical alkynes like hex-2-yne
The electrons in hex-2-yne shift so that a positive charge is present on the carbon attached to the alkyl group, allowing the carbocation to be stabilized by the +I effect of the alkyl group as well as the hyperconjugation of three -hydrogen atoms. As a result, Cl- of HCl attacks the carbocation, and the product are as follows:
Practice Problems
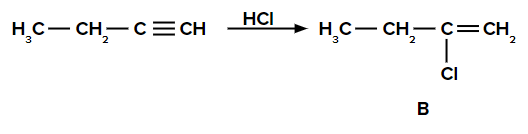
Q1. The addition of HCl to but-1-yne gives B and the addition of HI to B gives C. Find B and C.
Solution: The addition of HCl to alkynes follows the Markovnikov rule. In but-1-yne, the π electrons shift in such a way that the positive charge is present on the carbon attached to the alkyl (ethyl) group so that the carbocation is stabilized by the +I effect of the alkyl group and also by the hyperconjugation of two ⍺-hydrogen atoms.
Thus, Cl- of HCl attacks the carbocation, and the compound ‘B’ is obtained as follows:
The addition of HI to 2-chloro-1-butene (B) also follows the Markovnikov addition. Hence, the
product ‘C’ is given as follows:
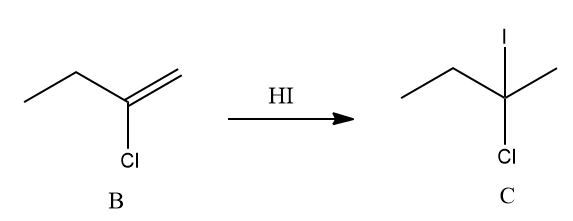
Hence, B is 2-chloro-1-butene and C is 2-chloro-2-iodobutane.
Q2. An electrophilic addition of HBr to A gives 1-Bromo cyclohexene and the addition of HCl gives B . Find A and B.
A. Cyclohexene and 1-Bromo-1-Chloro cyclohexene
B. Cyclohexyne and 1-Bromo-2-Chloro cyclohexene
C. Cyclohexyne and 1-Bromo-1-Chloro cyclohexene
D. Cyclohexyne and 1-Bromo-1-Chloro cyclohexane
Solution: The addition of HBr to A gives 1-Bromo cyclohexene, it must be an addition of halogen acid to alkyne cyclohexyne. In cyclohexyne, the π electrons shift in such a way that the positive charge is present on the carbon attached to the alkyl (ethyl) group so that the carbocation is stabilized.
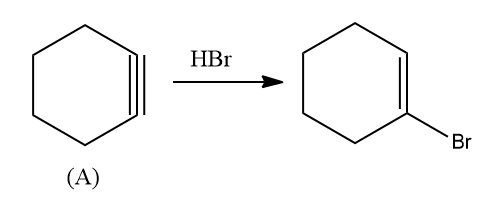
Hence, compound A is Cyclohexyne.
The addition of HCl to1-Bromo cyclohexene follows the Markovnikov addition. Hence, the product ‘B’ is given as follows:
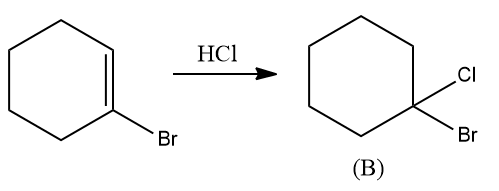
Hence, compound B is 1-Bromo-1-Chloro cyclohexane.
The correct answer is option (D).
Q3. Predict the product and its optical activity when successive electrophilic additions of HCl and HI to cyclopentyne gives the product.
A. 1-Chloro-2-iodo cyclopentane, optically active
B. 1-Chloro-2-iodo cyclopentane, optically inactive
C. 1-Chloro-1-iodo cyclopentane, optically active
D. 1-Chloro-1-iodo cyclopentane, optically inactive
Solution: The addition of HCl to cycopentyne gives 1-chloro cyclopentene. The addition of HI to1-chloro cyclopentene follows the Markovnikov addition and the product is given as follows:
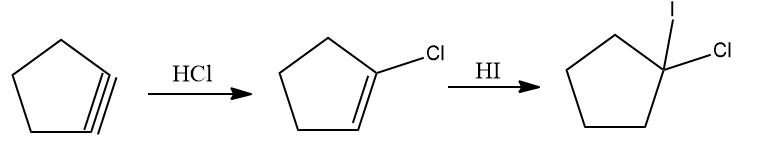
Hence, the product is 1-Chloro-1-Iodo cyclopentane. As this molecule possesses no chiral centre, (C1 has two identical alkyl groups) this molecule is optically inactive.
The correct answer is an option (D).
Q4. The addition of HCl to pent-2-yne gives
A. 2-chloro pent-2-ene
B. 3-chloro pent-2-ene
C. 2-chloro-3-chloropentane
D. 2,2-dichloropentane
Solution: The addition of HCl to alkynes follows the Markovnikov rule. In pent-2-yne, the π electrons shift in such a way that the positive charge is present on the carbon attached to the alkyl roup so that the carbocation is stabilized by the +I effect of the alkyl group and also by the hyperconjugation of three ⍺-hydrogen atoms.
Thus, Cl-of HCl attacks the carbocation, and the product is obtained as follows:
Hence, the product is 2-chloro pent-2-ene and the correct answer is an option (A).
Frequently Asked Questions
Q1. Why do alkynes exhibit addition reactions?
Answer: Because of the presence of loosely held pi-electrons, alkynes undergo addition reactions. Because alkynes contain a triple bond, halogens, water, and other substances can be added to them via the addition reaction.
Q2. What is an example of an electrophilic addition reaction?
Answer: Electrophiles are compounds that lack electrons. One sigma bond and two pi bonds exist between two carbon atoms in the alkyne. These are electron-rich species that form bonds with electrophiles to form electrophilic addition reactions.
Examples include halogenation and hydration reactions.
Q3. What is the function of the addition reaction?
Answer: Unsaturated compounds are added by a group of atoms or molecules to form saturated compounds in an addition reaction. It is widely used in the synthesis of various natural products and drugs. In industrial applications, vegetable oils are unsaturated compounds that are combined with hydrogen in the presence of a catalyst to form saturated compounds such as vegetable ghee.
Q4. Why do alkynes not prefer substitution reactions?
Answer: They do not substitute because it takes more energy to break existing bonds than it does to form new ones.
Q5. Does carbocation rearrangement occur in alkynes?
Answer: To form the expected addition product, an intermediate carbocation may rearrange or simply pick up a nucleophile. The situation is different with alkynes. Similar protonation of an alkyne bond would result in a very unstable vinyl cation, so this does not occur in most cases.


