-
Call Now
1800-102-2727
Haemoglobin: Structure, Types, Functions, Common Disorders, Practice Problems and FAQs
Every living cell needs a constant supply of materials like nutrients, oxygen and a simultaneous removal of waste materials like carbon dioxide, nitrogenous wastes etc. So various organisms develop different methods for this transport of materials depending on their structural organisation. We are aware that the cardiovascular system is the transport system in our body. It helps in the transport of all substances like nutrients, hormones, waste products, respiratory gases, etc. But have you ever wondered how these substances are transported or, which component of blood is mainly responsible for the transport of various substances?
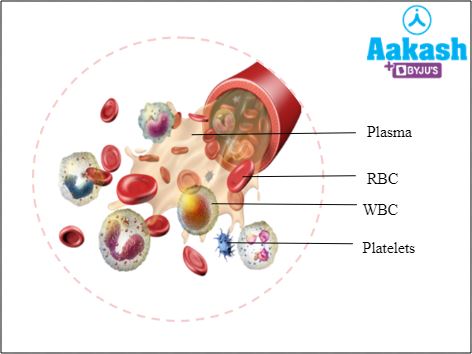
Fig: Components of blood
Well, nutrients, hormones, waste products (urea) and some amounts of respiratory gases (CO2 and O2) are carried by the plasma. But the majority of respiratory gases are transported by the RBCs. So, how does this happen?. This happens with the help of specialised haemoglobin molecules present in the RBCs. So let’s take a deep dive into the details of haemoglobin in this article.
Table of contents
- Blood
- Components of blood and their respective functions
- RBCs
- Haemoglobin
- Structure of haemoglobin
- Types of haemoglobin
- Functions of haemoglobin
- Common disorders related to haemoglobin
- Practice Problems
- FAQs
Blood
Blood is a specialised fluid connective tissue. It lacks fibres, and is composed of two components such as 55% fluid matrix (plasma) and 45% formed elements like RBCs, WBCs and platelets.
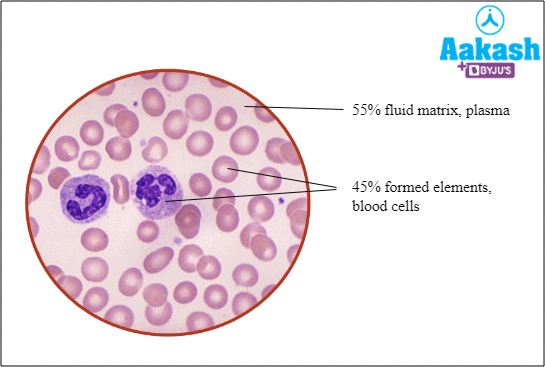
Fig: Composition of blood
Plasma
Plasma is a straw coloured or pale yellow viscous fluid which is alkaline in nature. It consists of 90 - 92% water and 8% solids. The solids include plasma proteins, nutrients (glucose, amino acids, fatty acids, phospholipids, cholesterol, nucleosides, fats and mineral salts), dissolved gases (oxygen, and carbon dioxide), waste products (urea, uric acid and creatinine), etc.
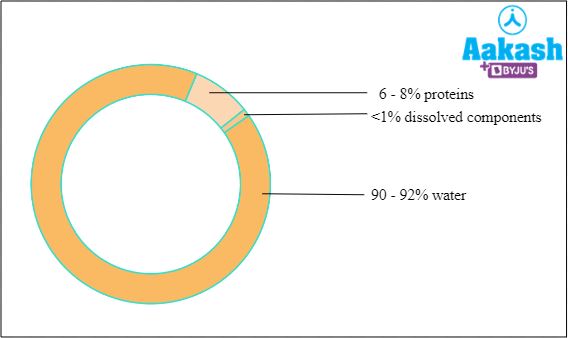
Fig: Composition of plasma
Components of blood and their respective functions
The following table shows the various components of blood and their respective functions:
Components |
Functions |
|
Plasma (water, proteins like fibrinogens, albumins and globulins, dissolved components like glucose, lipids, amino acids, dissolved gases, inorganic salts, etc.)
|
|
|
Platelets or thrombocytes
|
Help in blood clotting |
|
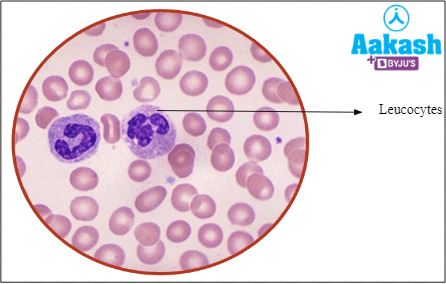
WBCs (White blood cells) or leucocytes
|
Responsible for providing immunity by fighting against disease causing pathogens that enter the body |
|
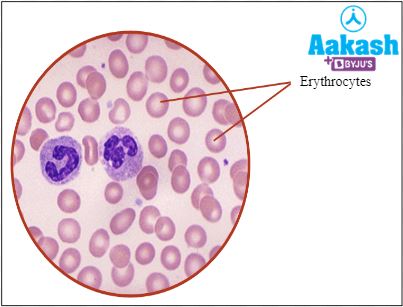
RBCs (Red blood cells) or erythrocytes
|
Helps in the transport of respiratory gases (O2 and CO2) |
RBCs
The red blood cells of animals like fishes, amphibians, reptiles and birds are oval and nucleated (contain a nucleus). In mammals, the mature RBCs are enucleated (do not contain a nucleus) and lack any organelles like mitochondria. In mammals, RBCs are circular or discoid and biconcave, except in camels and llamas (they possess oval or elliptical and nucleated RBCs).
The lack of a nucleus increases the surface area of RBCs so that they can carry a maximum number of oxygen molecules. The lack of mitochondria ensures that RBCs do not use up this oxygen for cellular respiration (breakdown of glucose to generate energy in the form of ATP molecules). RBCs break down glucose without using oxygen to generate energy (anaerobic oxidation).
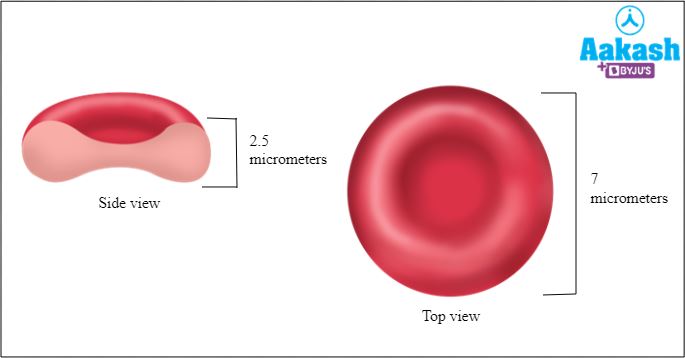
Fig: Shape and size of mammalian RBCs
Haemoglobin
The RBCs are packed with a special molecule called haemoglobin. It is the haemoglobin that is responsible for carrying respiratory gases (CO2 and O2).
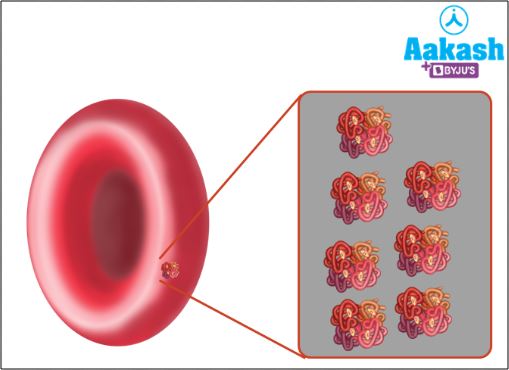
Fig: Haemoglobin molecules are present inside RBC
Structure of haemoglobin
Each RBC contains around 270 million haemoglobin molecules. Haemoglobin is a tetrameric protein. Each haemoglobin has two parts such as haem (non-protein part) and globin (protein part).
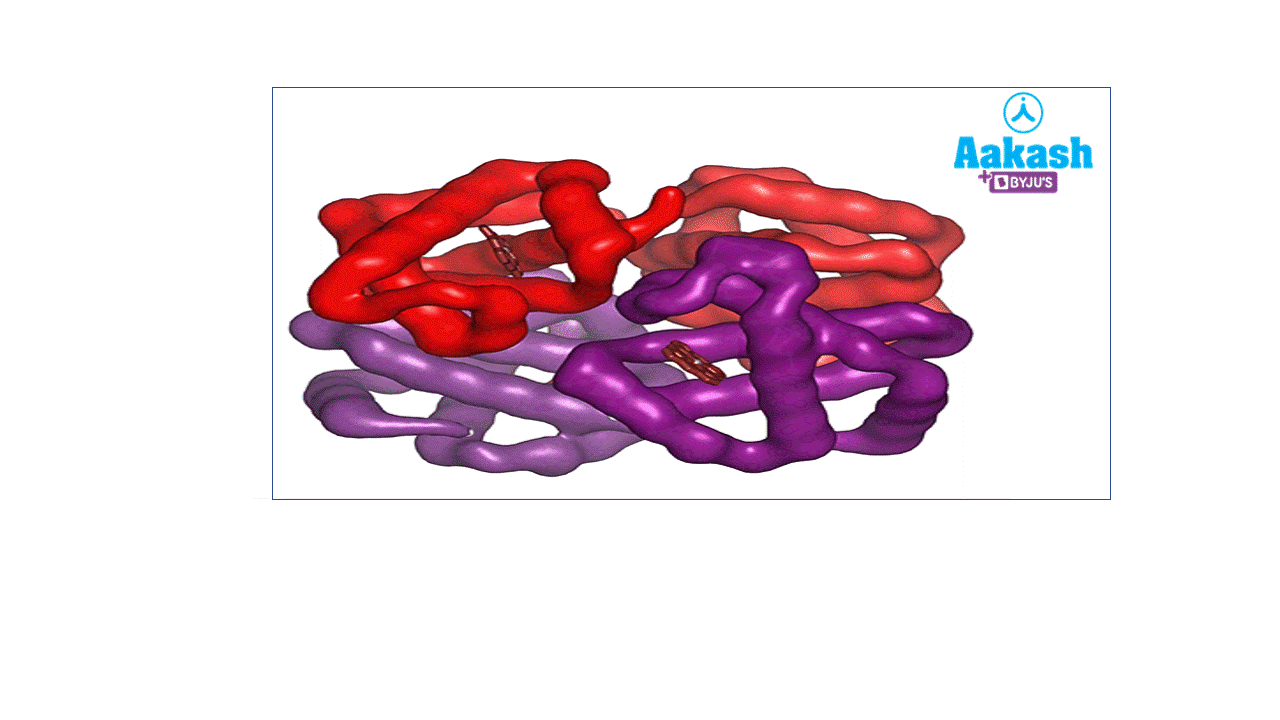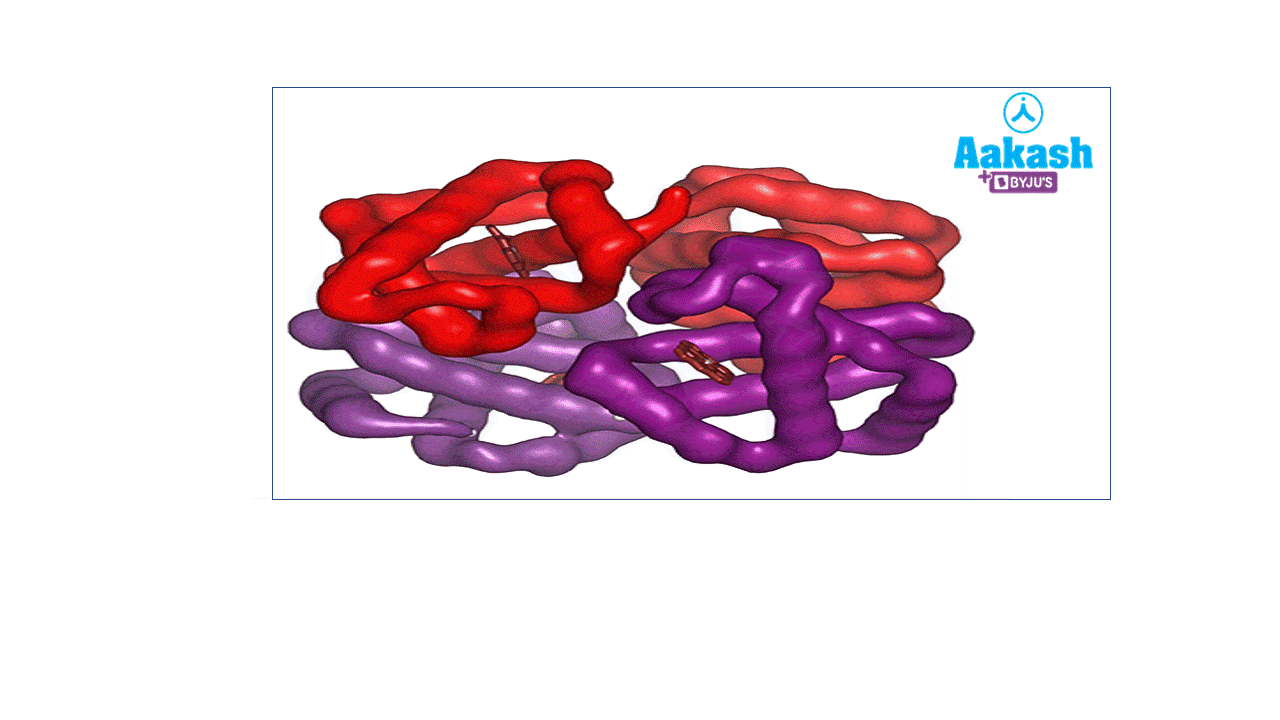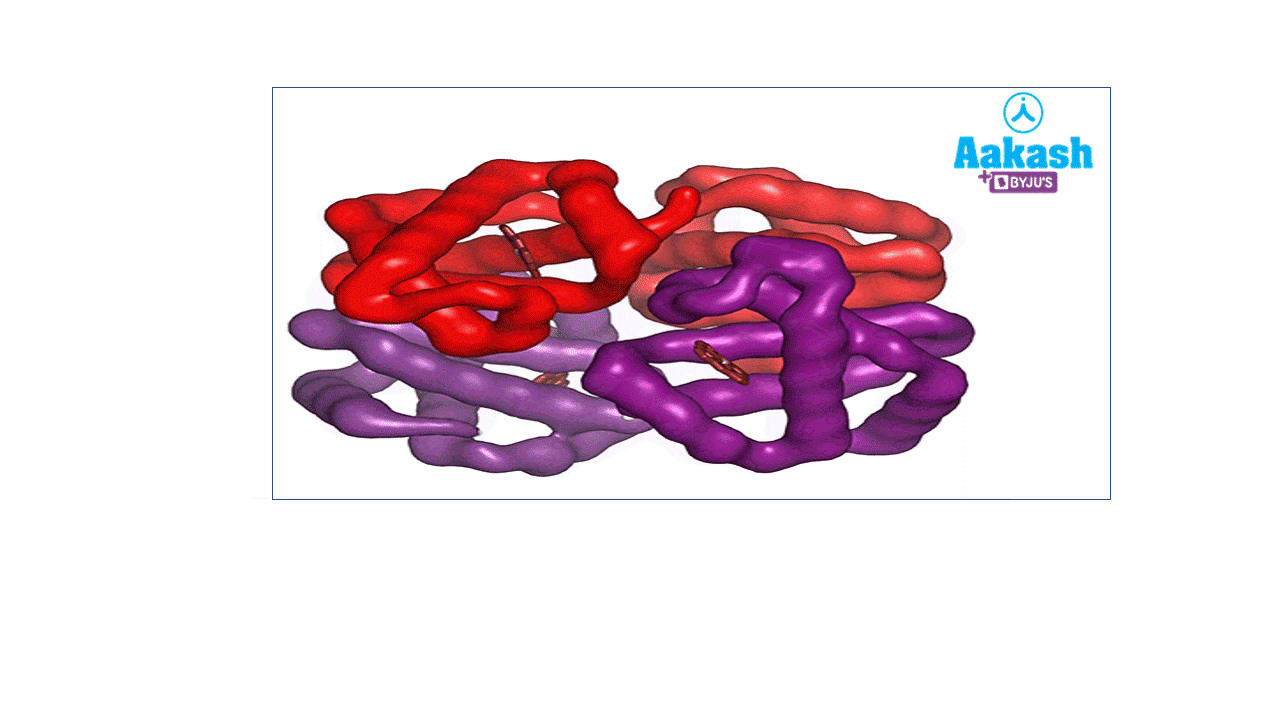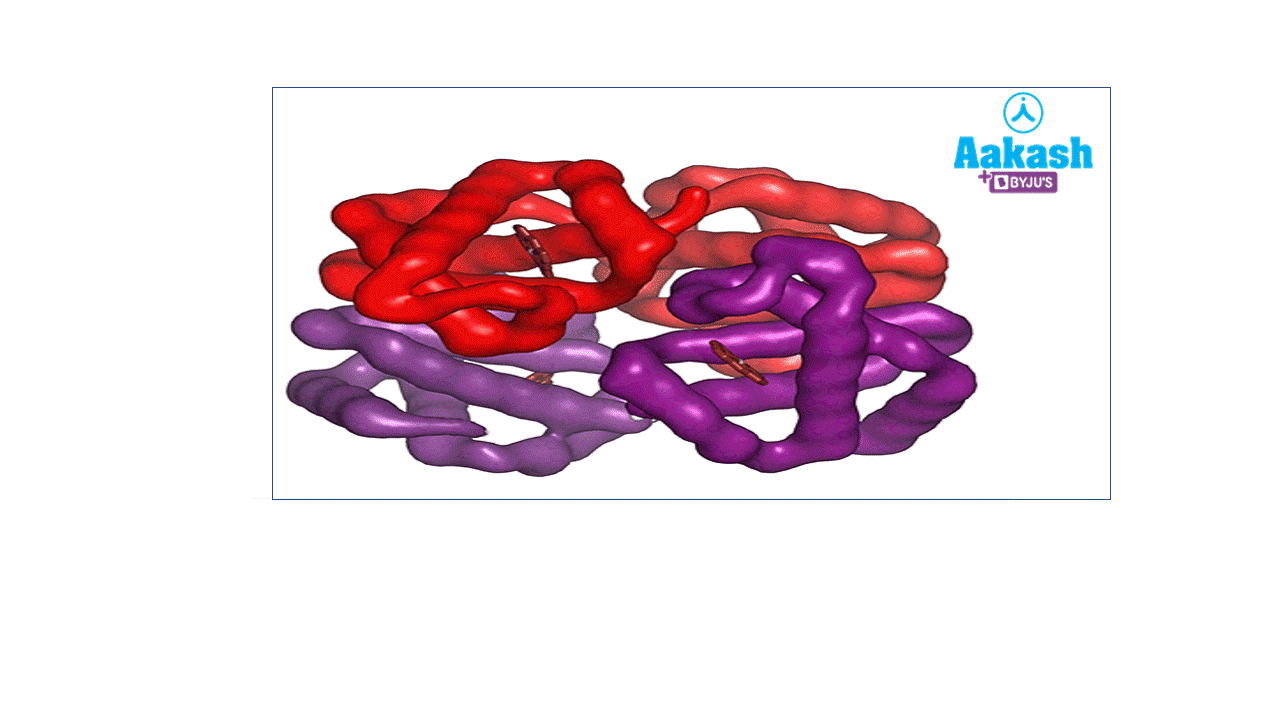
GIF: Haemoglobin
α globin chains (each with 146 amino acids)
Haem
It is the prosthetic group. Haem consists of a protoporphyrin ring and a central iron ( Fe2+) atom. The protoporphyrin ring is made up of four pyrrole rings linked by methane bridges. 4 haem groups are present in one haemoglobin. The presence of haemoglobin provides red colour to RBCs. Since, RBCs are the most abundant blood cells, our blood appears red in colour.
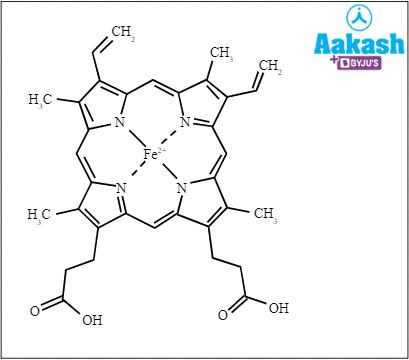
Fig: Haem structure
Globin
Each haemoglobin molecule is a tetramer made up of four polypeptide globin chains, 2 alpha and 2 beta chains.
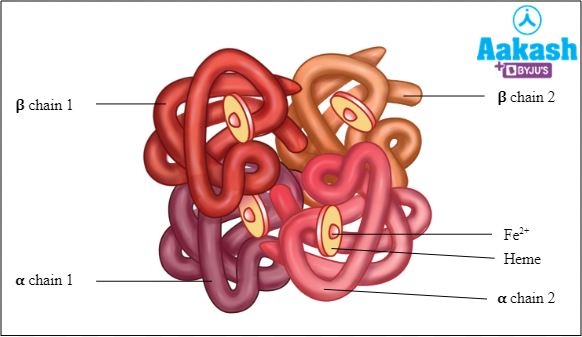
Fig: Structure of haemoglobin
Types of haemoglobin
Different types of haemoglobin are present in human beings. HbA, HbA2, HbE, HbF, HbS, HbC, HbH, and HbM are the most common types of haemoglobins. Healthy adults normally possess HbA and HbA2.
HbA
Haemoglobin A is also called adult haemoglobin, α2β2 or haemoglobin A1. It is the most common human haemoglobin tetramer which accounts for over 97% of the total red blood cell haemoglobin.
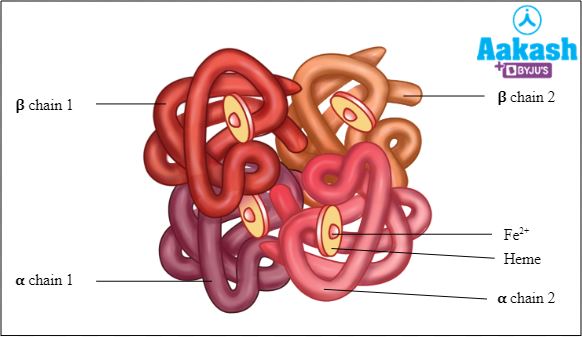
Fig: Structure of HbA
HbA2
Haemoglobin A2 is a normal variant of haemoglobin A. It consists of two alpha and two delta chains (α2δ2). It is found in human blood in low levels (1.5-3.1%). Amount of haemoglobin A2 increases in cases of beta-thalassemia.
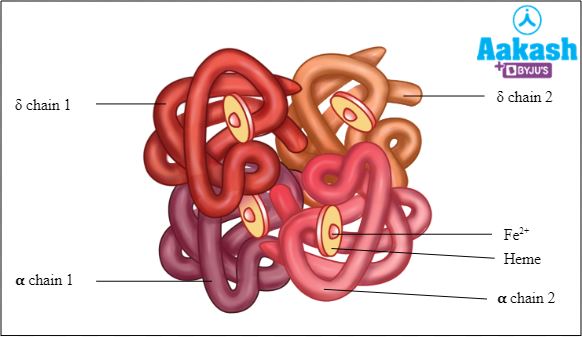
Fig: Structure of HbA2
HbE
Haemoglobin E is a β-haemoglobin variant. It occurs at high frequencies throughout many Asian countries. It is produced at a slightly reduced rate and is commonly seen in β thalassemia cases.
HbF
Foetal haemoglobin is a dominant form of haemoglobin seen in the foetus during gestation. It is produced by erythroid precursor cells from 10 to 12 weeks of pregnancy till the first six months of postnatal life. It contains two alpha and two gamma subunits (α2γ2).
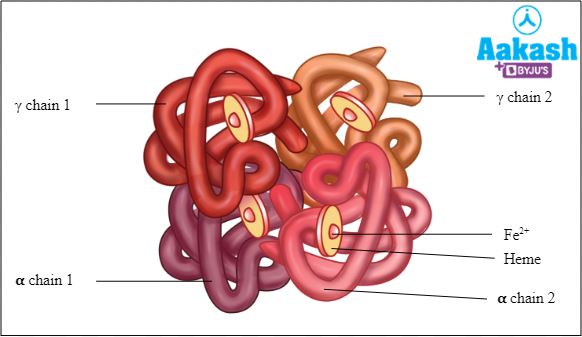
Fig: Structure of HbA2
HbS
It is an abnormal form of haemoglobin which is associated with sickle cell anaemia. The red blood cells have a sickle or crescent shape in this condition. These cells easily break down and block small blood vessels.
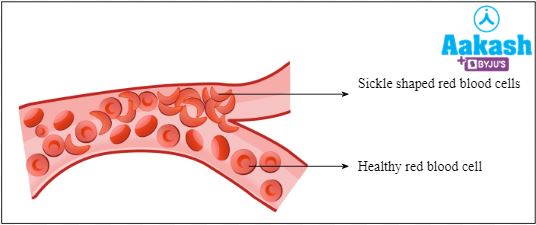
Fig: Sickle-shaped red blood cells
HbC
It is an abnormal form of haemoglobin seen associated with hemolytic anaemia. The symptoms of this condition are milder than sickle cell anaemia.
HbH
Haemoglobin H is present in persons with α-thalassemia. The excess beta globin chains in them combine with each other to form haemoglobin H. Hence α-thalassemia is also called haemoglobin H disease. HbH can make up to 40% of circulating haemoglobin in affected individuals. HbH disease is the most severe and non-fatal form of α-thalassemia.
HbM
Methemoglobin is normally called HbM and is formed by a variety of mutations in the α, β, and γ-globin genes. HbM is formed by the above congenital changes in the haemoglobin synthesis and metabolism. It is also caused by acute adverse drug reactions. This can lead to cyanosis and hemolytic anaemia.
Functions of haemoglobin
Haemoglobin molecules have two major functions as follows:
- Provide pigmentation to blood.
- Transportation of respiratory gases.
Pigmentation to blood
The presence of iron containing heme groups in the haemoglobin gives the red colour to RBCs. The colour scale method by the WHO or World Health Organization is a semi-qualitative method used to check the pigmentation of blood. It is an inexpensive method used for estimating the haemoglobin concentration from a drop of blood. Once the blood is taken it is compared with a colour scale. The colour scale consists of a small card with six shades of red colour that represent haemoglobin levels at 4, 6, 8, 10, 12, and 14 g/dL, respectively. This method is recommended in anaemia patients.
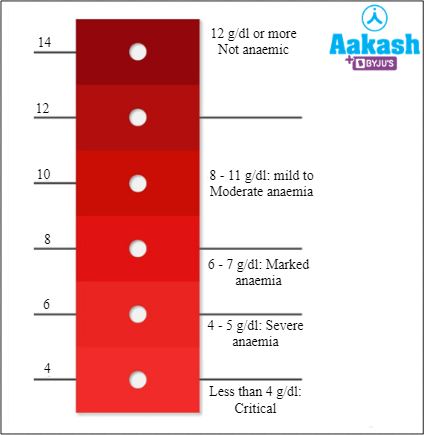
Fig: Colour scale method
Transportation of respiratory gases
Blood is the fluid connective tissue that transports oxygen from the respiratory organ to different tissues and carbon dioxide from tissues to the respiratory organs.
Transportation of oxygen
Each Fe2+ can bind to one oxygen molecule. Thus, one haemoglobin molecule binds to four O2 molecules.
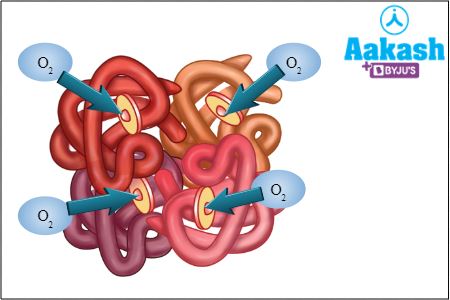
Fig: Oxyhaemoglobin
Only one gas can bind to haemoglobin at a time. The binding depends on the partial pressure of the gas. This type of binding of any gas with haemoglobin is weak and it ensures that the gas will be released at its destination. For example, the partial pressure of oxygen in alveoli is higher than in blood. Therefore, as the blood in capillaries reaches the level of alveoli, oxygen diffuses from alveoli into the blood and binds with haemoglobin, forming oxyhaemoglobin. This RBC then reaches the tissues via circulation and releases oxygen that now diffuses from blood to tissues.
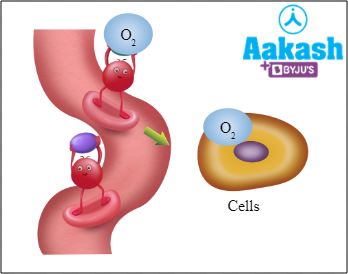
Fig: Oxyhaemoglobin delivering O2 to cells
Transportation of carbon dioxide
Each polypeptide chain in haemoglobin can bind one carbon dioxide molecule at its amino end. Thus, one haemoglobin can bind four CO2 molecules.
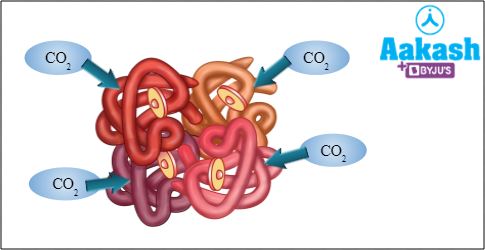
Fig: Carbaminohaemoglobin
The partial pressure of carbon dioxide is higher in tissues than in blood. So, CO2 diffuses from tissues into the blood and binds to haemoglobin, forming carbaminohemoglobin. When this RBC reaches the alveoli via blood circulation, CO2 is released and it diffuses from blood into alveoli, and finally it is exhaled out.
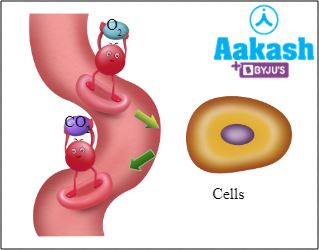
Fig: Hb delivering O2 to cells and carrying CO2 away from cells
Common disorders related to haemoglobin
The levels of haemoglobin in a normal healthy individual is 12 - 16 gms/100 ml of blood. Abnormally high or low levels of haemoglobin can cause problems.
Anaemia
This disorder is characterised by abnormally low levels of healthy RBC count to carry enough oxygen to the tissues.
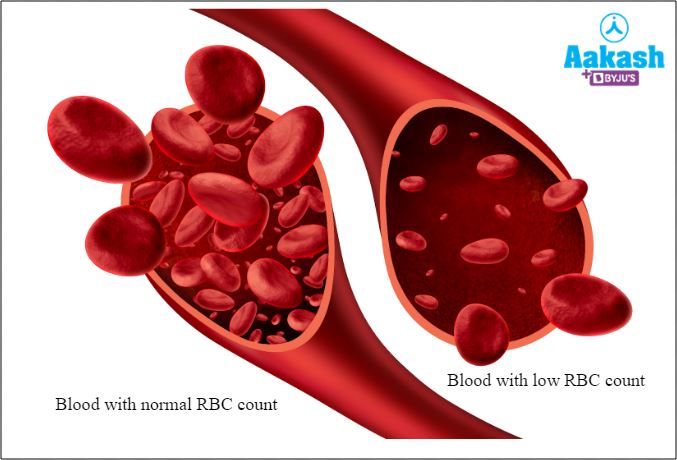
Fig: Anaemia
Causes of anaemia
The following are the common causes of anaemia:
- Iron deficiency (Haemoglobin will not be produced).
- Vitamin B12 deficiency (Healthy RBCs are not formed).
- Sickle cell anaemia (Sickle shaped RBCs produced).
- Low RBC count.
- Haemolysis (RBCs destroyed faster than produced).
- Excessive bleeding due to injury or accident.
In any case, a low RBC count indicates a low haemoglobin level that leads to hypoxia where body tissues do not receive enough oxygen.
Common symptoms of anaemia
A person suffering from anaemia will have the following symptoms:
- Pale or yellow skin
- Irregular heartbeats
- Chest pain
- Fatigue
- Weakness
- Dizziness
- Shortness of breath
- Cold hands and feet
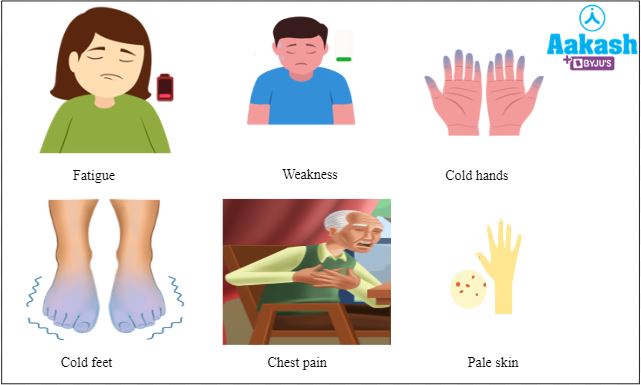
Fig: Common symptoms of anaemia
Prevention of anaemia
The anaemic condition can be prevented by following a healthy diet. One must include the following in their diet to prevent anaemia:
- Iron rich foods like spinach, meat, beans, dried fruits, etc.
- Folate rich foods like green peas, peanuts, kidney beans, bread, cereals, rice, etc.
- Vitamin B12 rich foods like meat, dairy products, etc.
- Vitamin C rich foods which include Citrus fruits like lemon and oranges, tomatoes, broccoli, peppers etc.

Fig: Suggested food items to prevent anaemia
Abnormally high haemoglobin
This can happen under normal circumstances like if the person smokes, or lives at a higher altitude like in the mountains.
At high altitudes, the atmospheric pressure is low, thus the partial pressure of oxygen in the atmosphere is also low. When a person migrates from plains to mountains, they feel nauseous, weak, fatigued and have shortness of breath. This happens because enough oxygen is not available to the tissues. But with time, the body increases the RBC count to ensure more RBCs are available to bind to atmospheric oxygen and thus the tissues receive enough oxygen. The kidney of such individuals releases a hormone called erythropoietin in response to the decreased oxygen. Erythropoietin signals bone marrow to produce more RBCs. More RBCs means more haemoglobin (Hb). If there is more Hb, our blood can carry more oxygen.
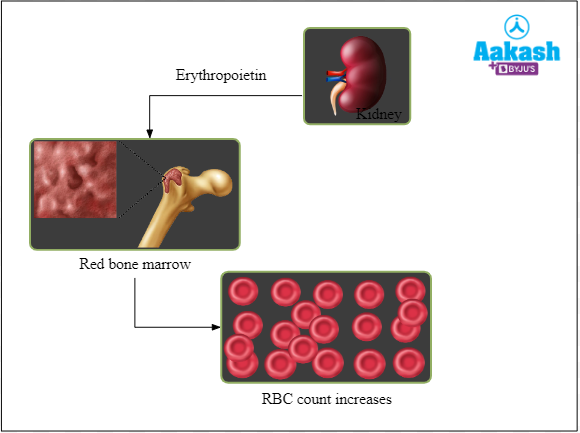
Fig: Release of erythropoietin increases the production of RBCs
A high haemoglobin can also be a result of serious health conditions like congenital heart disease in adults, COPD (chronic obstructive pulmonary disease), heart failure, kidney or liver cancer, polycythemia vera (a type of blood cancer where the bone marrow produces excessive RBCs).
Haemoglobin C disease
In this condition lysine is substituted by glutamate at the 6th position in the beta-globin chain. Due to this mutation Hb C is less soluble than Hb A and forms hexagonal crystals in the peripheral smear. This mutation can exist in homozygous conditions (HbC HbC) where the person suffers from mild chronic haemolysis, splenomegaly (enlargement of the spleen) and jaundice. This condition can also exist in heterozygous conditions (HbA HbC) and the person is phenotypically normal with no symptoms.
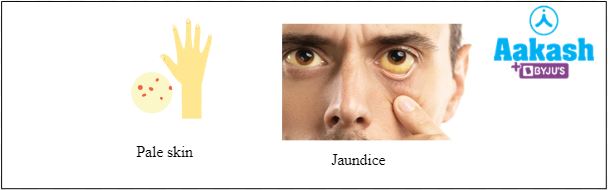
Fig: Common symptoms of haemoglobin C disease
Hemoglobinopathy
It is the most common type of inherited disease in the world. It includes all the genetic disorders of haemoglobin. There are two main categories of haemoglobinopathy, these are thalassemia (alpha and beta) and abnormal variants of haemoglobin (HbS, HbE and HbC).
Thalassemia
It is a genetic disorder that results because of mutations in the globin chains of haemoglobin. Alpha thalassemia results because of mutation in the alpha-globin chain, whereas, beta thalassemia results because of insufficient production or absence of the beta-globin chain.
Common symptoms of thalassemia
A person suffering from thalassemia can be diagnosed by looking for the following signs:
- Pale skin and jaundice.
- Bronze skin in severe conditions.
- Deformed facial or other bones, called chipmunk face.
- Iron deposition in cardiac cells that can lead to arrhythmia.
- Gallstones.
- Enlarged liver and spleen, called hepatosplenomegaly.
- Iron deposits in various organs thereby affecting their functions.
- Slow growth of children and delayed puberty.
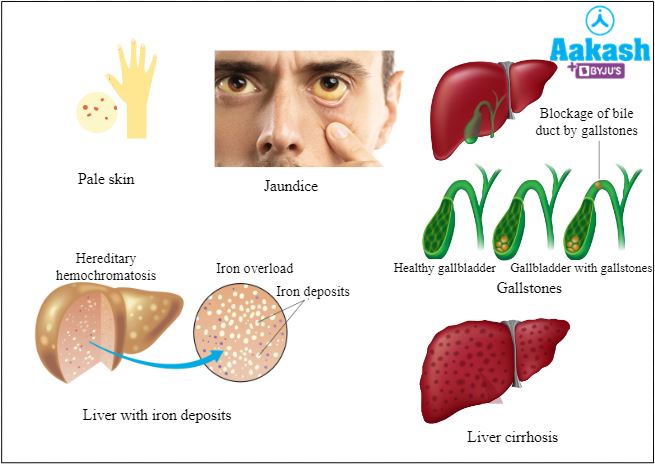
Fig: Few symptoms of thalassemia
Methaemoglobinaemia
This condition could be due to genetic mutation in haemoglobin molecules or a result of exposure to various oxidant drugs and toxins. In any case, the oxygen carrying capacity of haemoglobin is reduced because Fe2+ (ferrous) gets oxidised to Fe3+ (ferric) state. Fe3+ can not bind to oxygen. Therefore, this condition leads to functional anaemia.
Sickle cell anaemia
It is an autosomal-linked recessive trait that is heritable. It occurs because of a defect in the beta-globin chain as glutamate is substituted by valine on the 6th position. This leads to a change in the shape of RBC from circular to sickle-shaped. A person can be homozygous (HbS HbS) or heterozygous (HbA HbS) for this condition. Homozygous people have 100 % RBCs of sickle shape. Whereas, heterozygous people have 50% circular RBCs and 50% sickle-shaped RBCs.
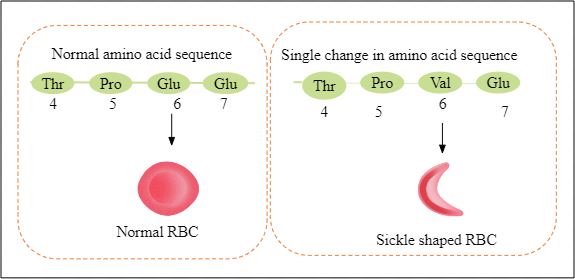
Fig: Mutation in sickle cell anaemia
These sickle-shaped RBCs are rigid and sticky. They have reduced oxygen carrying capacity and also they can not travel via thin capillaries.
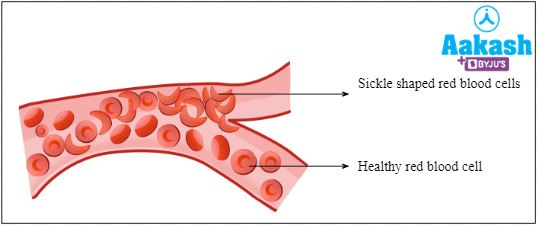
Fig: Movement of normal and sickle shaped RBCs in blood vessel
Practice Problems
Q1: Decrease in plasma fibrinogen will likely affect ___________.
a. clot formation
b. osmotic balance
c. oxygen carrying capacity
d. immune functions
Solution: Fibrinogen is an inactive blood clotting protein, upon activation leads to blood clot formation. Albumin is required for maintaining osmotic balance. Haemoglobin present inside RBC is responsible for carrying oxygen from the lungs to body tissues. Globulin proteins are responsible for proving immunity. Hence, the correct option is a.
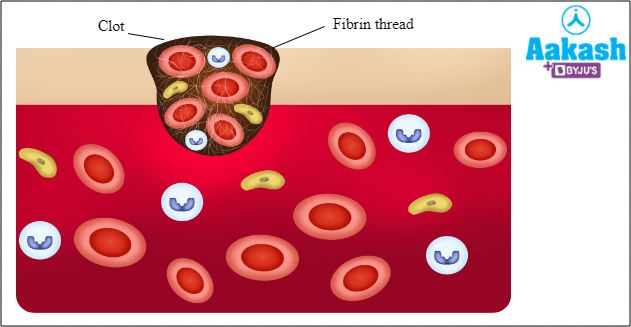
Fig: A blood clot
Q2: Which of the following statements is true for the RBCs of man?
a. They are round and nucleated
b. They are oval and enucleated
c. They are biconvex and nucleated
d. They are biconcave and enucleated
Solution: Mammalian RBCs are circular, biconcave and lack a nucleus, except in camels and llamas. Their RBCs are oval and contain a nucleus. Hence, the correct option is d.
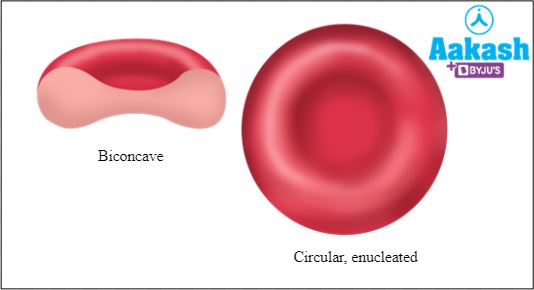
Fig: Shape of mammalian RBCs
Q3: In which of the following conditions does the body not produce enough RBCs?
a. Haemolytic anaemia
b. Sickle cell anaemia
c. Aplastic anaemia
d. Altitude sickness
Solution: Aplastic anaemia is a rare condition when the body is not able to produce enough RBCs due to bone marrow damage either present at birth or can happen due to radiation and chemotherapy. Hence, the correct option is c.
Q4: Which of the following functions can not be performed by a mature RBC?
a. Aerobic respiration
b. DNA synthesis
c. Anaerobic respiration
d. Both a and b
Solution: A mature RBC lacks a nucleus and mitochondria. It can not perform DNA synthesis and aerobic respiration. Rather, it performs anaerobic oxidation. Hence, the correct option is d.
FAQs
Q1: How are haemoglobin levels measured?
Answer: Haemoglobin levels can be measured using Sahli’s method. It involves use of a haemoglobinometer. This method involves converting haemoglobin into acid haematin. Result is calculated and written in g/dL of blood.
Q2: What are the similarities and differences between the molecular structures of haemoglobin and a chlorophyll molecule?
Answer: The haemoglobin molecule is the respiratory pigment in vertebrates which gives red colour to red blood cells. It has 4 polypeptide chains and 4 haem groups. The haem group is the prosthetic group. Haem consists of a protoporphyrin ring and a central iron ( Fe2+) atom. The protoporphyrin ring is made up of four pyrrole rings linked by methane bridges.
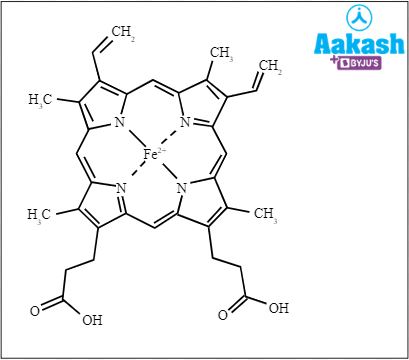
Fig: Haem structure
Chlorophyll is the pigment that provides green colour to the plant parts. It is also the pigment that captures the light energy needed for photosynthesis. It consists of a porphyrin derivative and a magnesium ion.
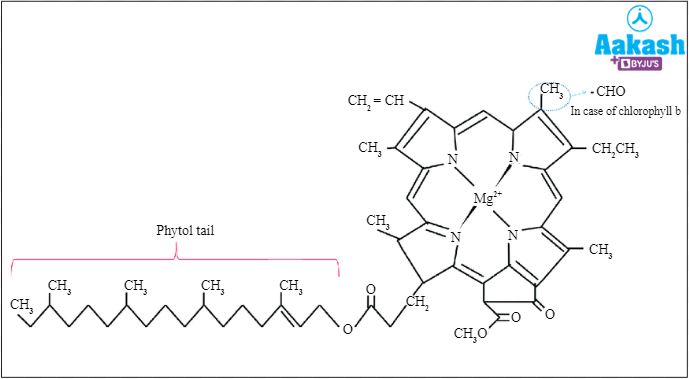
Fig: Chlorophyll structure
Thus, both molecules are similar in having a porphyrin ring. But, haemoglobin has iron ions in the centre of the ring, whereas the chlorophyll molecule has magnesium ions in the centre of the ring.
Q3: Which are the different types of WBCs or leucocytes?
Answer: WBCs are of two types such as granulocytes and agranulocytes.
Granulocytes - These cells contain cytoplasmic granules that take up stains. If the granules take up acidic stains, the WBC is an eosinophil or acidophil and appears red in colour under the microscope. If it takes up the basic stain, the WBC is basophil and appears blue in colour. If the granules take up both stains, the WBC appears purple and is a neutrophil.
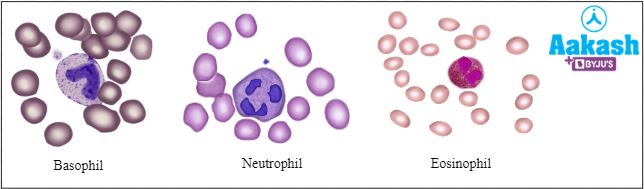
Fig: Granulocytes
Agranulocytes - These cells do not contain any cytoplasmic granules. They are of two types such as monocytes, which contain a horse-shoe-shaped nucleus and lymphocytes, which contain a large circular nucleus.
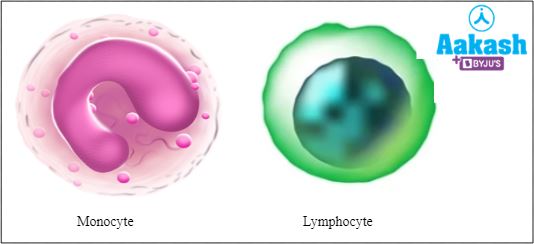
Fig: Agranulocytes
Q4: Why do camels have different RBCs as compared to other mammals?
Answer: Camels live in deserts, where the temperature is very high and they do not get water easily. They have nucleated RBCs, due to which the shape of their red blood cells are thicker in the middle and have an elliptical shape. This is advantageous to camels as this RBC can now easily move through the blood vessels, like tiny capillaries of the camel, even when the camel is dehydrated or the plasma levels are low.

Fig: Camel
Youtube link:NEET 2022 - Physiological Process of Respiration, Breathing and Exchange of Gases (Class 11 Biology)
Body Fluids and Circulation Class 11 Biology | Blood & Its Components, Blood Coagulation | NEET Prep