-
Call Now
1800-102-2727
Preparation of Amines: Reduction of - Nitro, Nitriles, Isocyanides, Amides, Azides and Oximes, Ammonolysis of Alkyl Halides, Gabriel Phthalimide Synthesis, Rearrangement Reactions
Well, we all know one of the most important macronutrients for our body is protein, it helps in building muscle and also responsible for many physiological processes occurring in our body. If we stop eating foods or supplements that provide us protein then automatically, the body will have to break down muscle fibres to release the protein it needs to survive if there is not enough protein in the diet. Hence, next time don’t skip pulses which have these proteins from your regular diet.
Have you ever wondered how proteins are made?
You might be thinking of amino acids. Yes, amino acids are the building blocks of proteins but amino acids are produced from amines. Amines are useful in many other ways.
Let’s understand how we can prepare amines from different methods and from different sources.
Table of Content
- Amine
- Classification of Amines
- Reduction of Nitro Compounds
- Reduction of Nitriles
- Reduction of Isocyanides
- Reduction of Azides
- Reduction of Oximes
- Ammonolysis of Alkyl Halides
- Practice Problems
- Frequently Asked Questions
Amine
Amine is a chemical compound derived from ammonia (NH3). In other words, amines are ammonia derivatives. Amines are organic nitrogen compounds that contain a nitrogen atom with a lone pair. In most amines, hydrogen atoms of ammonia are replaced with an aryl or alkyl group.
Classification of Amines
Classification of amines is based on two factors that are
- Based on the types of groups attached to N
- Based on the number of groups attached to N
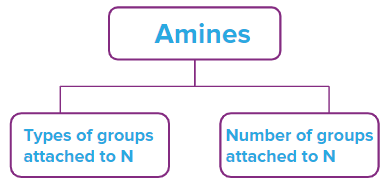
Amines, based on the types of groups attached to N
Amines are classified into three categories based on the types of groups attached to N
- Alkylamines
- Arylamines
- Heterocyclic amines
- Alkyl Amines
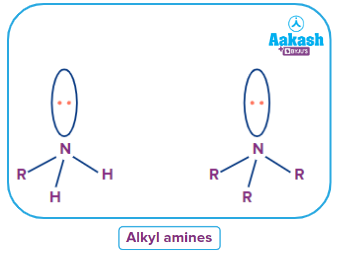
An alkyl amine is a substance in which the nitrogen atom of an amine is joined to an alkyl group. Alkyl amines are created when any of the ammonia's three hydrogen atoms is changed for an alkyl.
For example, Methylamine and propylamine
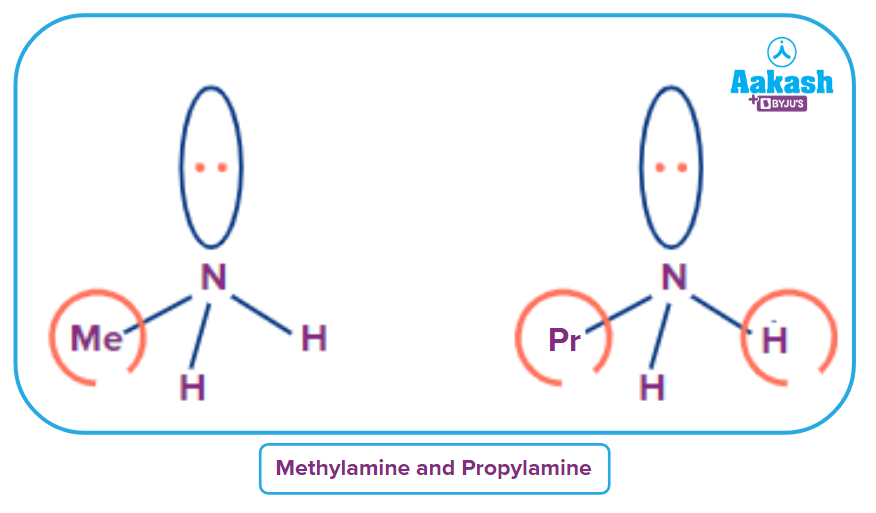
- Aryl Amines
In aryl amines, any of a category of amines in which aromatic groups are used in place of one or more of the hydrogen atoms in ammonia.
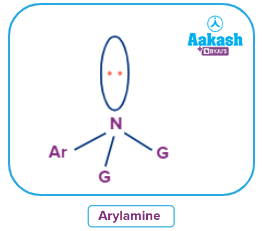
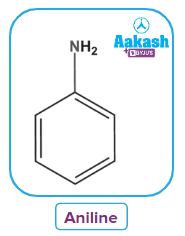
- Heterocyclic Amines
These are cyclic compounds containing one or more nitrogen as a part of the ring structure.
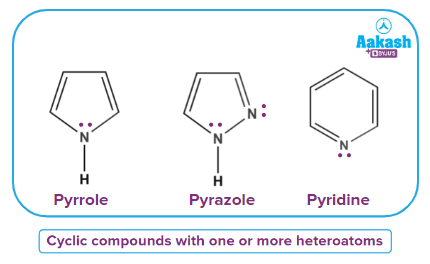
Amines, based on the number of groups attached to N
Amines are classified into three groups: primary (10), secondary (20), and tertiary depending on how many hydrogen atoms in ammonia are changed for an alkyl or aryl group (30). When one hydrogen atom is replaced, amines with the formula R-NH2 or primary amines (10) are created. Alkyl/aryl groups can replace two of the three hydrogen atoms to form secondary amines. If an alkyl or aryl group is substituted for all three hydrogen atoms, tertiary amines are created.
- Primary Amines
When an alkyl or aromatic group replaces one of the hydrogen atoms in ammonia, a primary amine is created. Amino acids and methylamine are examples of primary alkyl amines, whereas aniline is an example of a primary aromatic amine.
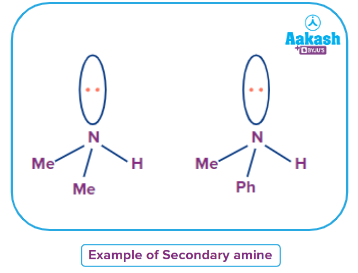
- Secondary Amine
An amine in which the amino group has a direct link to two carbons of any hybridization, which takes place when an organic molecule with two amine groups. Secondary amines are also called enamine.
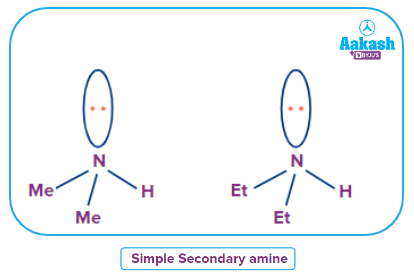
Simple secondary amine
An amine where all alkyl or aryl groups attached to the nitrogen atom are the same then it is called a simple secondary amine.
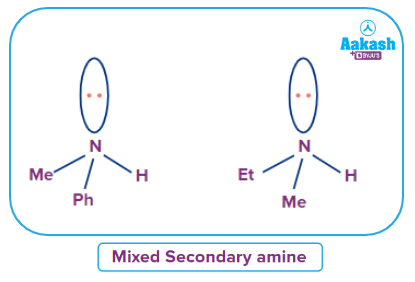
Mixed Secondary Amine
An amine where all alkyl or aryl groups attached to the nitrogen atom are not the same then it is called a simple secondary amine.
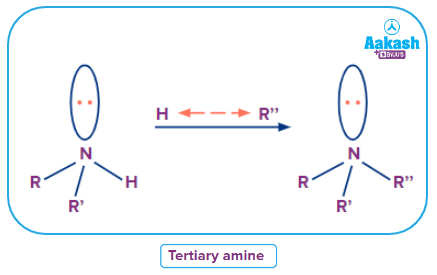
- Tertiary Amine
An amine in which three carbons of any hybridization, but not of carbonyl group carbons, are directly linked to the nitrogen atom, is a tertiary amine structure. or any three-carbon groups besides carbonyl.
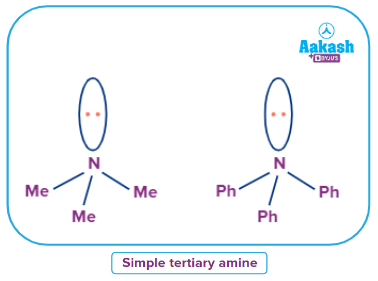
Simple Tertiary Amine
An amine Where all alkyl/aryl groups attached to N are the same then it is called a simple tertiary amine.
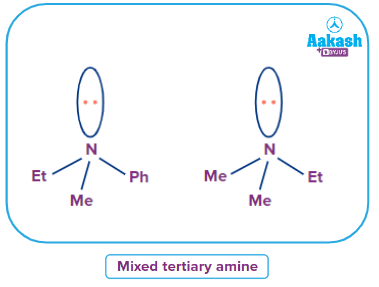
Mixed Tertiary Amine
An amine Where all alkyl/aryl groups attached to N are not the same then it is called a simple tertiary amine.
Methods of Preparation of Amines
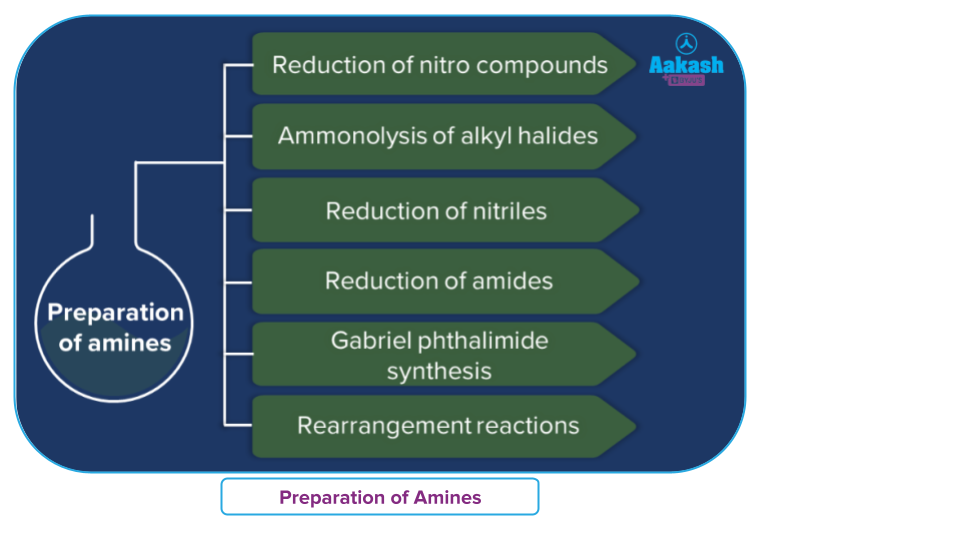
Amines are generally prepared by the following methods:
- Reduction of nitro compounds
- Reduction of nitriles
- Reduction of Isocyanides
- Reduction of Amides
- Reduction of Azides
- Reduction of Oximes
- Ammonolysis of alkyl halides
- Gabriel phthalimide synthesis
- Rearrangement reaction
- Reduction of Nitro Compounds
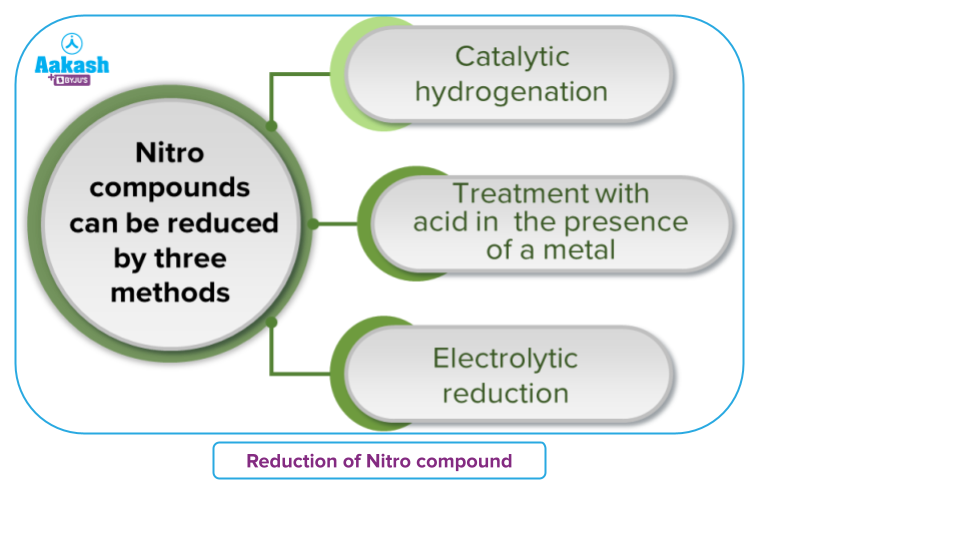
Both aliphatic and aromatic primary amines can be easily prepared by the reduction of the corresponding nitro compounds. This reduction can be achieved in a number of ways:
- Catalytic hydrogenation
- Treatment with acid in the presence of a metal
- Electrolytic reduction
- Catalytic hydrogenation
Nitro alkanes on passing hydrogen in the presence of Raney Ni, finely divided Pt or Pd or Pd/C, as a catalyst at room temperature gives primary amines.
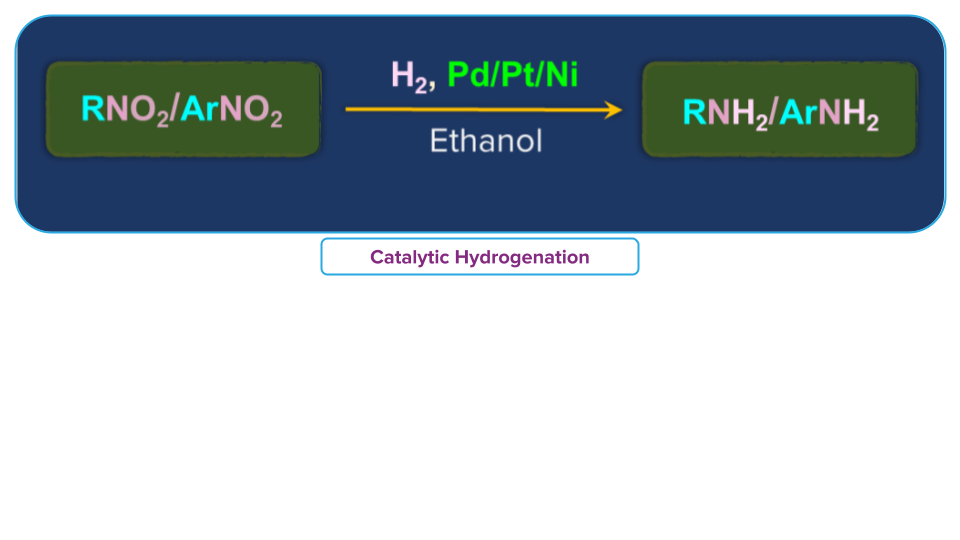
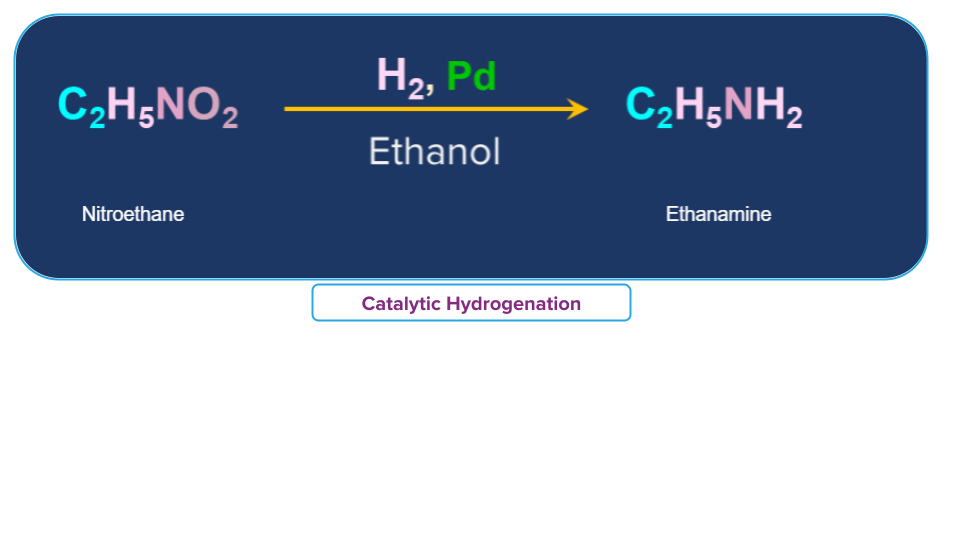
For example
Nitro ethane (C2H5NO2) in presence of finely divided Pt or Pd or Pd/C gives ethanamine (C2H5NH2).
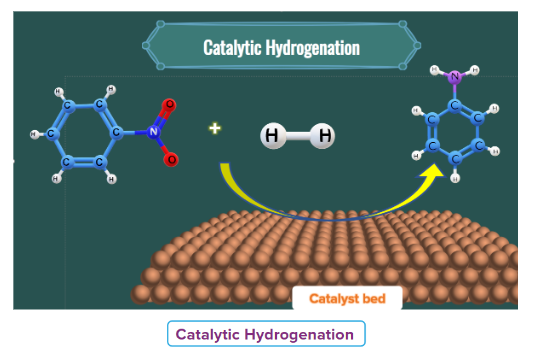
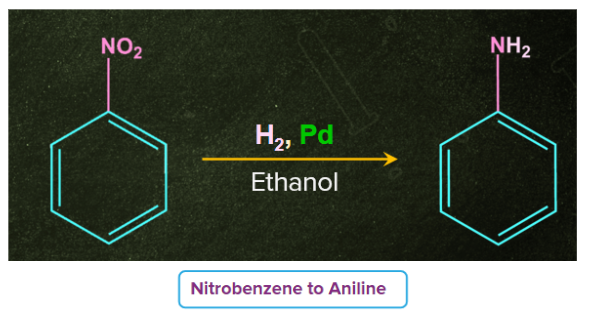
Another example can be considered where nitrobenzene is been converted to aniline by passing H2 gas in presence of finely divided Pt or Pd or Pd/C.
- Treatment with acid in presence of metal
Nitroalkane is converted to primary amine in presence of an active metal such as, Sn, Zn, etc, and conc. Hydrochloric acid.
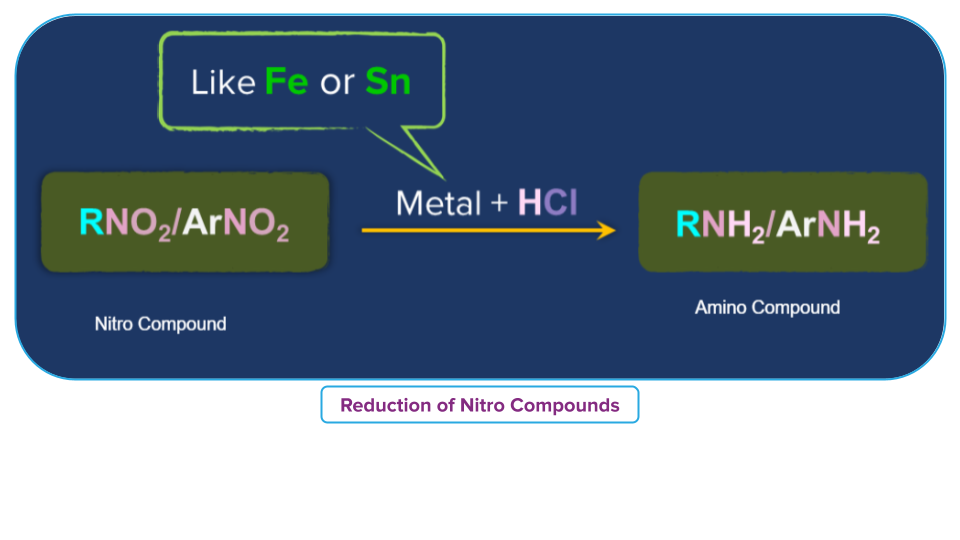
For example,
- Nitrobenzene in presence of a metal such as tin or iron and with concentrated HCl reduces to aniline.
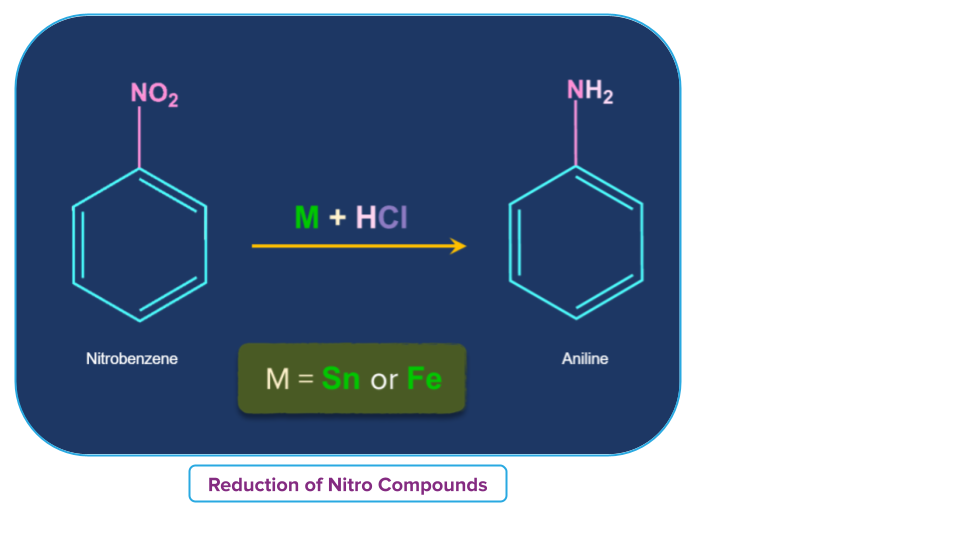
A mixture of SnCl2 and Conc. HCI has also been used for the reduction of aromatic nitro compounds. Reduction with iron scrap and hydrochloric acid is preferred over Sn/HCI because FeCl2 formed gets hydrolysed to release hydrochloric acid during the reaction. Thus, only a small amount of hydrochloric acid is required to initiate the reaction.
The reduction of nitro compounds to primary amines is one of the most convenient methods for the preparation of aromatic primary amines since they cannot be prepared from the corresponding aryl halides on treatment with ammonia. The required nitro compounds can be easily prepared by the nitration of arenes.
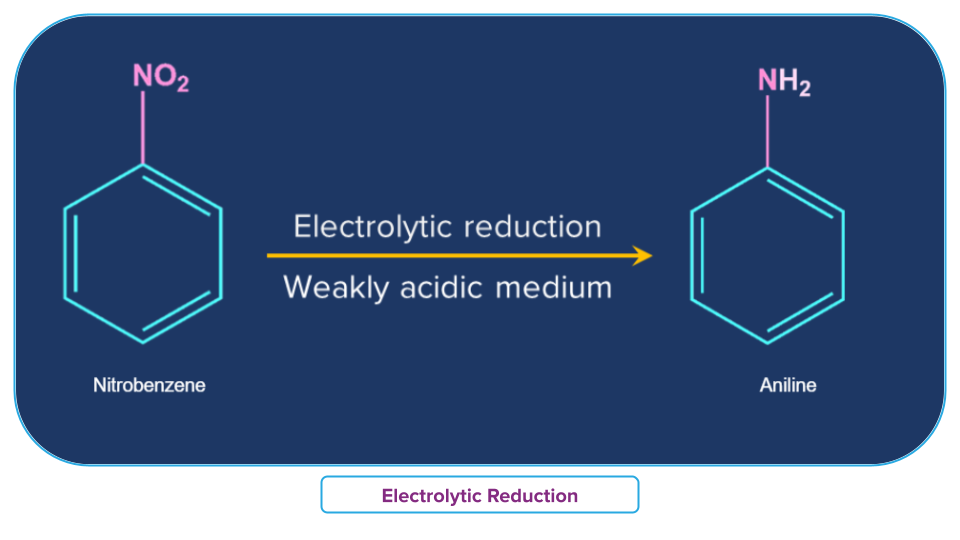
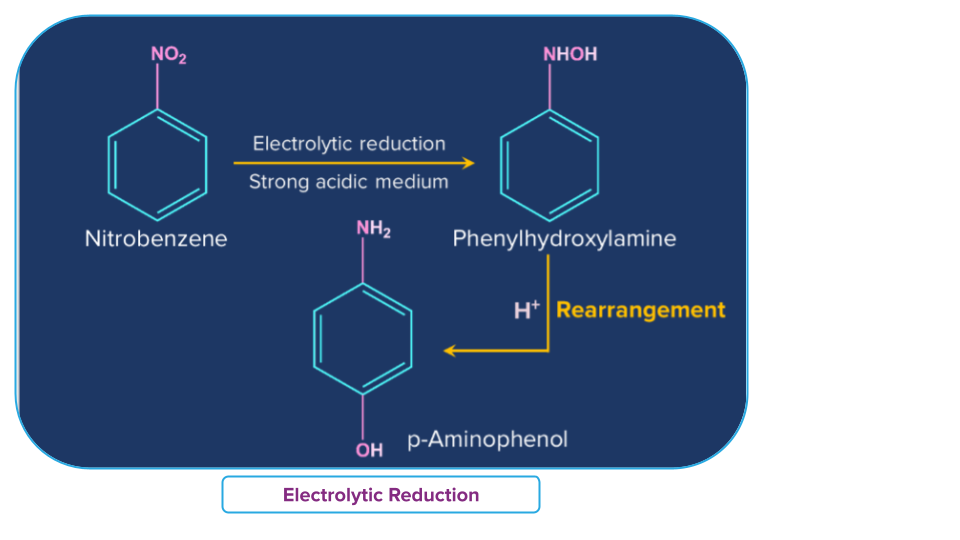
- Electrolytic reduction
Nitrobenzene on electrolytic reduction yields aniline in a weakly acidic medium, but p-aminophenol in a strongly acidic medium. Various mono and di-nuclear reduction products, such as Azoxybenzene and Azobenzene, are obtained in an alkaline medium.
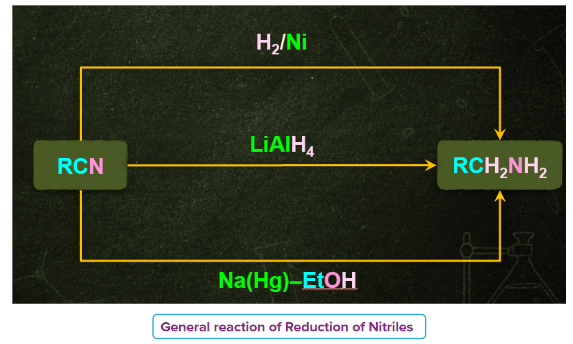
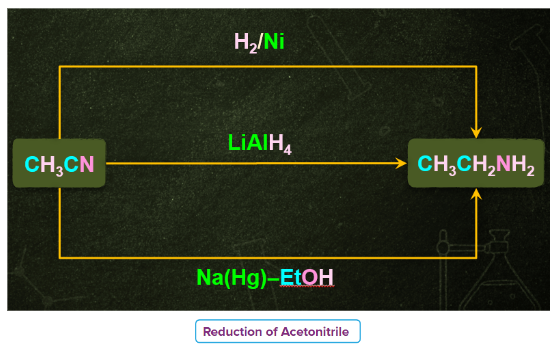
2) Reduction of Nitriles
- In organic chemistry, nitriles, commonly known as cyano compounds.. The carbon atom is connected to a functional group known as the cyano group, which is denoted by the functional group (-C N).
- By reducing with lithium aluminum hydride nitriles can be converted to several primary amines.
- Nitriles which are obtained from aldehydes undergo partial reduction to form imine, this reaction is commonly known as Stephen reduction. Imine on further reduction converts into primary amine in the presence of reducing agents that is Sn/HCl.
- This reaction is used for the preparation of amines containing one carbon atom more than the starting compound.
3) Reduction of Isocyanides or Carbylamines
- Reduction of isocyanides or carbylamines gives secondary amines i.e., N- methylamines.
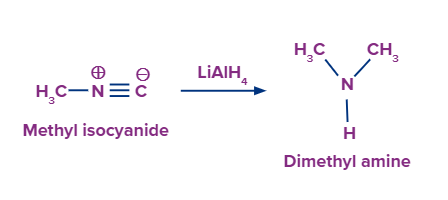
This method can be used only for the preparation of 2° amines in which one of the alkyl groups is always methyl.
- Hydrolysis of isocyanides or carbylamines
Hydrolysis of isocyanides or carbylamines with dilute mineral acids (but not with alkalies) always give primary amines (containing one carbon less than the starting isocyanide) along with formic acid.
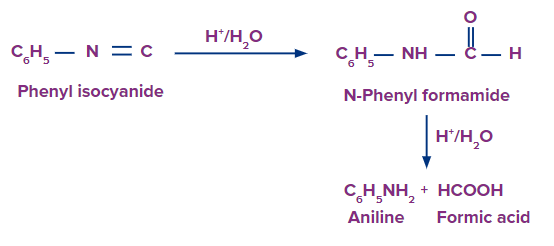
The negative charge present on the carbon atom in isocyanides initially attracts electrophiles (i.e., H+) but repels nucleophiles (OH- ion). As a result, isocyanides are hydrolysed only by acids but not by alkalies. Once a proton gets attached to the negatively charged carbon atom, the tendency of this carbon atom to attract a nucleophile increases due to the presence of a positive charge on the nitrogen atom and thus facilitates hydrolysis as shown below:
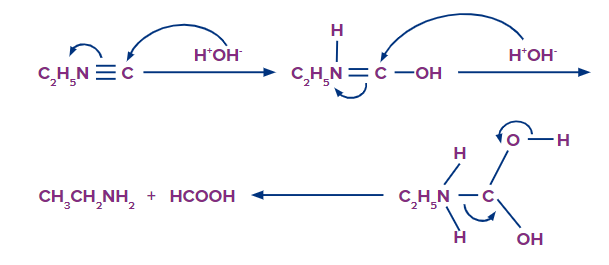
4) Reduction of Amides
Primary, secondary and tertiary amines can be prepared by the reduction of the corresponding amides with lithium aluminium hydride (LiAIH4). Secondary and tertiary amines can be prepared by the reduction of secondary and tertiary amides respectively, For example,
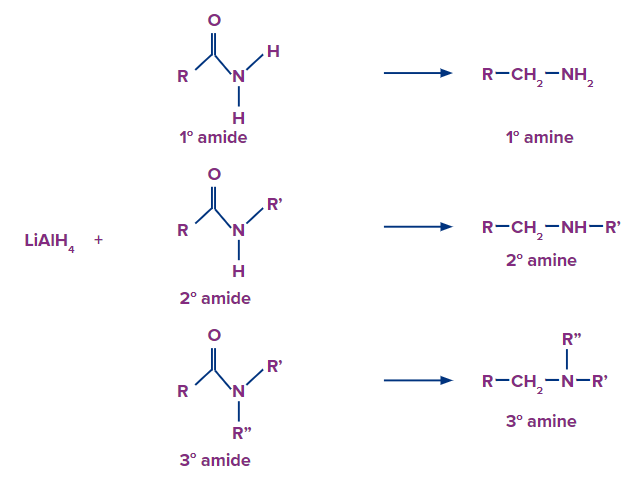
id
5) Reduction of azides
- Any chemical compound in the class of azides that has three nitrogen atoms arranged in a group is symbolized by the symbol (-N3). Azides are thought to be generated from hydrazoic acid (HN3), a salt like sodium azide (NaN3
- or an organic derivative in which the hydrogen atom of hydrazoic acid is replaced by a hydrocarbon group, as in alkyl or aryl azide (RN3), or by an acyl (carboxylic acid) group, like in acyl azide.
- Gabriel phthalimide synthesis is used for the production of primary amine but during the process of reaction high-temperature condition is needed for the cleavage of phthalimide with hydrazine.
- Note the triple bond between nitrogen and nitrogen in the resonance form above. Organic azides can be converted to primary amines and released N2 when handled with a reducing agent LiAlH4 or even catalytic hydrogenation (Pd/C, H2). This provides a very practical pathway from alkyl halides to primary amines.
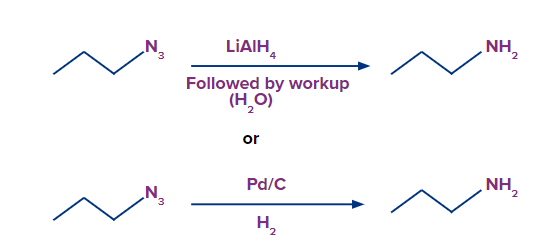
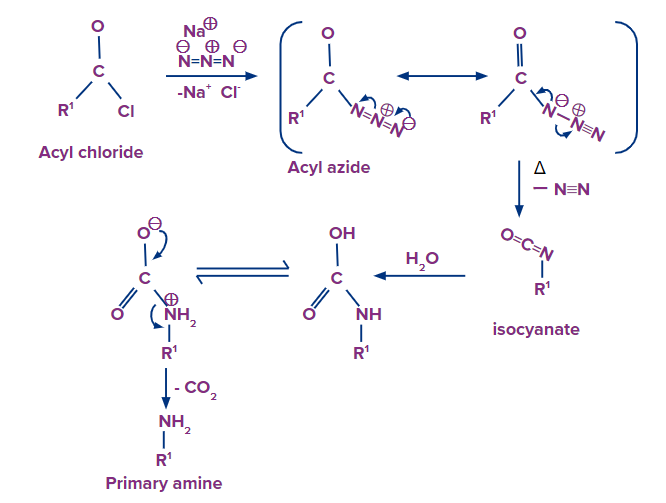
When acyl chloride is treated with sodium azide, acyl azide/acid azide is formed.
Acid azides can be converted to isocyanides when acid azide is heated in presence of nickel. Isocyanides further convert into a primary amine, when isocyanide is dehydrated and a rearrangement reaction follows up.
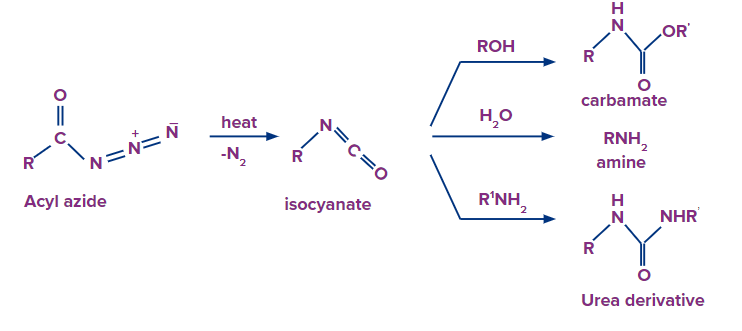
The reaction is commonly called a Curtis rearrangement reaction. The mechanism of this reaction is discussed below.
The mechanism of this reaction involves the following steps
Formation of Isocyanate
Step -1
Migration of carbon atom to displace the nitrogen gas leaving group on an adjacent nitrogen atom.
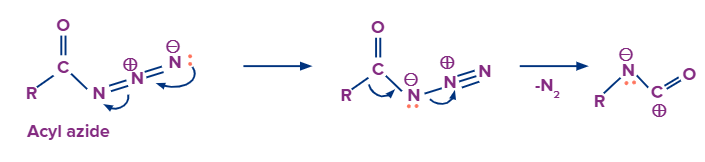
Step-2
Formation of the isocyanate by donation of a lone pair of electrons from nitrogen atom to the carbocation
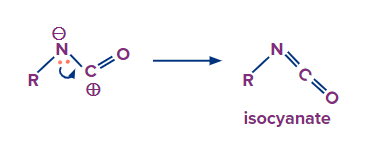
Transformation of isocyanate
Addition of water molecule results in the formation of carbamic acid from isocyanate. Carbamic acid is generally unstable and it quickly loses carbon dioxide to give a primary amine.
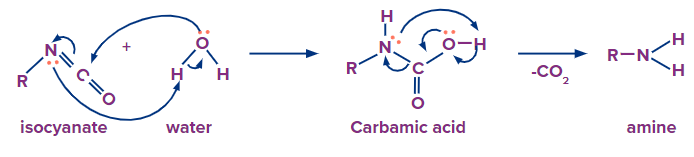
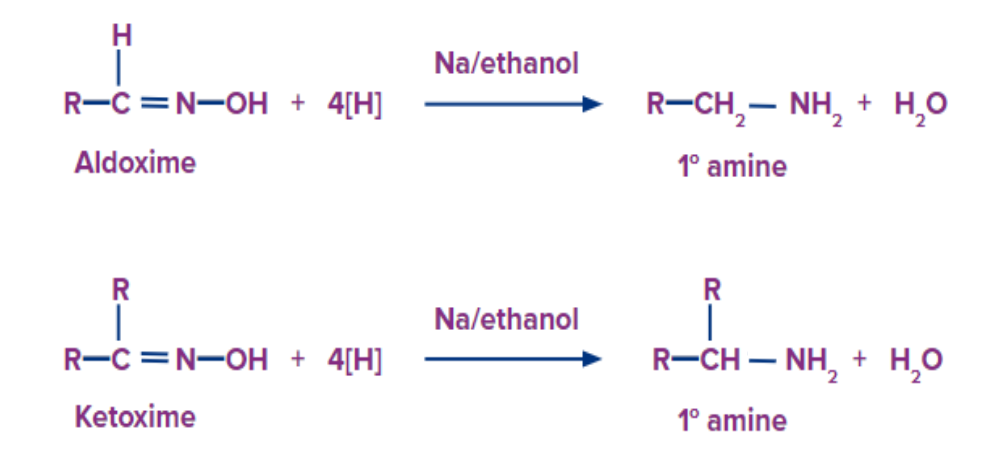
6) Reduction of Oximes
Oximes are chemical substances that fall within the imine class and have the general formula R1R2C=N O H. Where R1 is the organic side chain and R2 is hydrogen, creating an aldoxime or a molecule similar to another class of organic compounds like ketoxime. A category of substances that are extremely closely linked is the O-substituted group of oximes.
- Oximes, which are made from hydroxylamine, a ketone, and an aldehyde, are also known as nitrogen-containing organic molecules.
- These compounds can also be created via the isomerization of nitroso compounds or by the reaction of nitro compounds with hydrogen-donating reagents.
- Aldoximes, or oximes made from aldehydes, can also be dehydrated to create nitriles.
- The other chemical reactions include turning it into amides and turning it into amines by using hydrogen or other reducing agents. By reacting it with potent acids, this is produced.
Oxime has a two-sided chain-like structure with a carbon atom at its centre. The two side chains are completely different from one another. A hydroxyl group is present in one of the two chains.
Aldicarb oxime, aldoxime, dimethylglyoxime, ketoxime, methoxime, etc. are a few examples of oximes.
Oxime is created and water molecules are eliminated when an aldehyde or ketone combines with hydroxylamine (NH2OH) in a weakly acidic media.
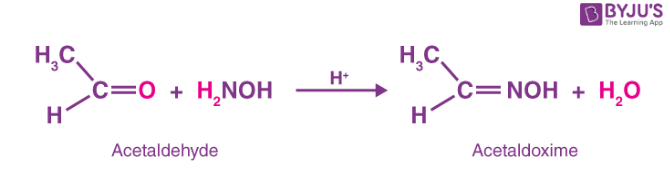
- When acetaldehyde reacts with hydroxylamine it produces acetaldoxime
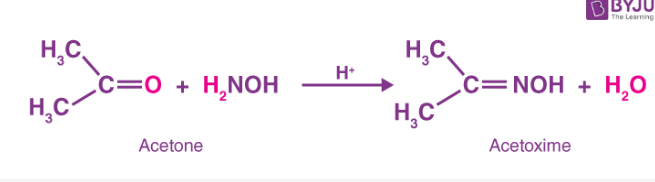
- When acetone reacts with hydroxylamine it produces acetoxime.
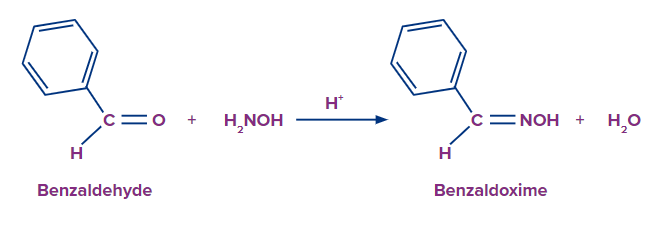
- Hydroxylamine and benzoaldehyde combine to generate benzoaldoxime and water.
Now the oxime is reduced in presence of a reducing agent to give primary amine
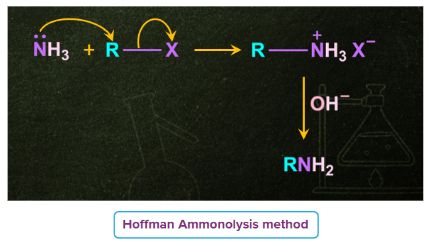
7) Ammonolysis of Alkyl Halides
When an alkyl halide is heated with alcoholic ammonia in a sealed tube under pressure at 373 K, a combination of primary, secondary, and tertiary amines and a quaternary ammonium salt are formed. Ammonolysis of alkyl halides is the name given to the process in which ammonia breaks the C–X bond.
This method is of very limited synthetic application because multiple alkylations that can occur resulting in secondary, tertiary and quaternary compounds
8)Gabriel Phthalimide Synthesis
This is a very convenient method for the preparation of pure aliphatic and aryalkyl primary amines, Phthalimide on treatment with ethanolic KOH gives potassium phthalimide which on heating with a suitable alkyl or aralkyl halide gives N-substituted phthalimides. These upon subsequent hydrolysis with dil. HCl under pressure or with alkali give primary amines.
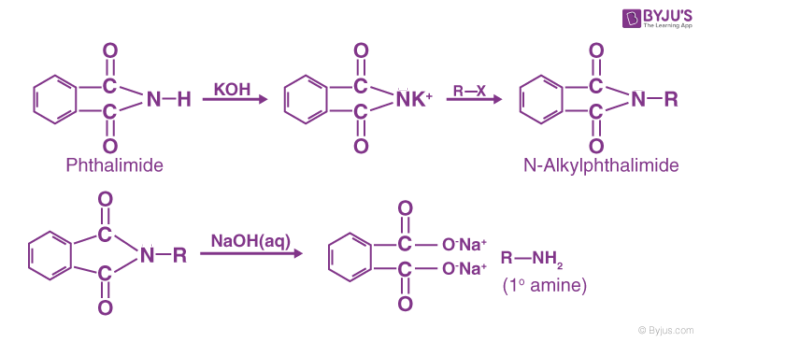
Phthalic acid can again be converted into phthalimide and used over and over again. Similarly, benzylamine can be prepared by using benzyl chloride and glycine (NH2CH2COOH) by using ethyl chloroacetate in place of ethyl iodide in the above reaction.
Aromatic 1° amines such as aniline, toluidines, etc. cannot be prepared by this method because ary1 halides do not undergo nucleophilic substitution reaction with potassium phthalimide under mild conditions.
The acidic/basic hydrolysis of N-alkylphthalimides is often slow. Therefore, hydrazinolysis (cleavage by hydrazine) of N- alkylphthalimides is a more convenient and efficient method for obtaining 1° amine using Gabriel synthesis. For example,
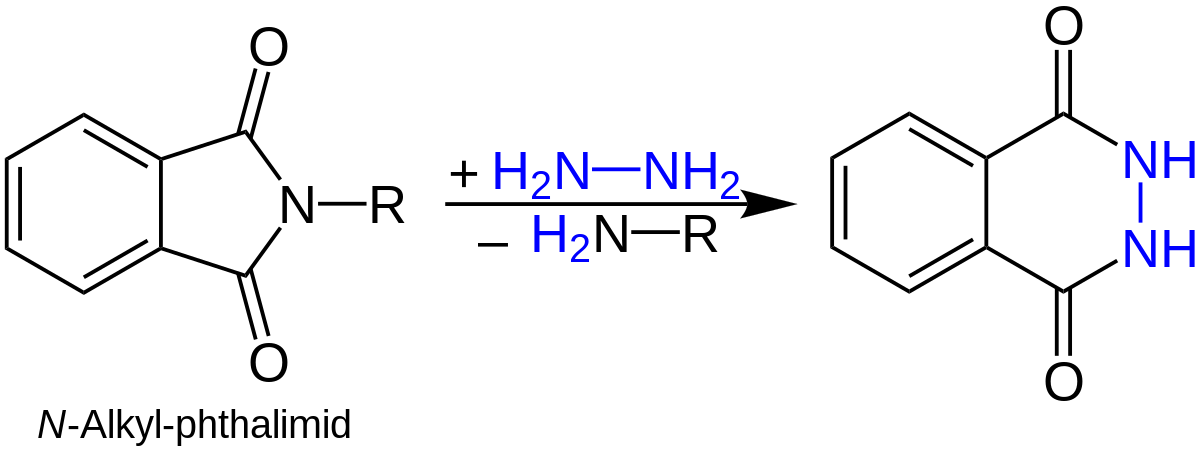
99
99
9) Hofmann degradation of Amides. (Hofmann Bromamide Reaction)-
When a primary amide is treated with an aqueous or ethanolic solution of potassium hydroxide and bromine (or potassium hypobromite, KOBr), it gives a primary amine which has one carbon atom less than the original amide:
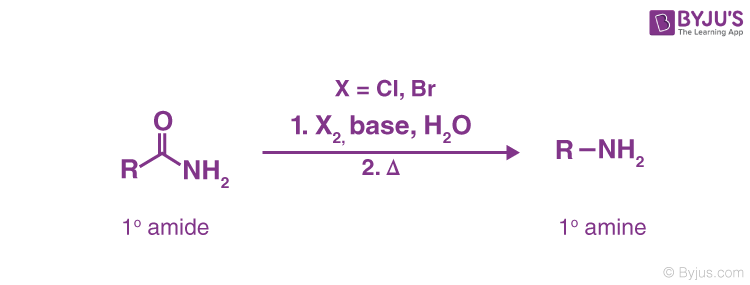 be
be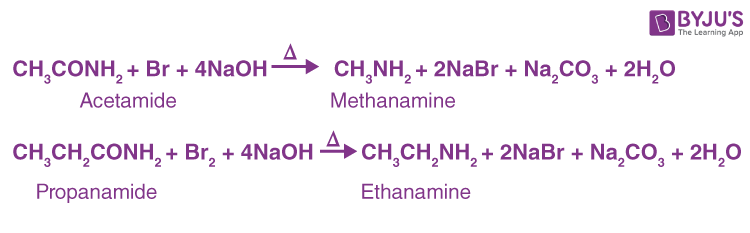
This reaction is widely used for stepping down or descent of homologous series.
Practice Problems
Q1. The preferred reagent for converting nitroethane to ethylamine is which of the following?
A. H2/Pt
B. Sn/HCl
C. Zn/HCl
D. Fe/HCl
Answer: (D)
Solution: Because FeCl2 is produced in this reaction and is hydrolyzed to release HCl, reducing nitroalkanes with iron scrap and HCl is preferable. Therefore, starting the reaction only requires a little quantity of HCl.
Q2. What kind of amine is produced when alkyl halides undergo ammonolysis?
A. Primary
B. Secondary
C. Tertiary
D. All of the above
Answer: (D)
Solution: All three forms of amines—primary, secondary, and tertiary—, as well as a quaternary ammonium salt, are produced when an alcoholic solution of ammonia is heated with alkyl halides.
Q3. Which option below wouldn't be a viable choice for converting an aryl nitro compound to an amine?
A. Sn and HCl
B. Fe and HCl
C. LiAlH4-ether
D. H2/Pt
Answer: (C)
Solution: Even though lithium aluminium hydride is a reducing agent, it doesn't interact with nitro compounds. In contrast to that, it reduces nitriles to produce the corresponding primary amines.
Q4. What compound is created when nickel that has been finely divided and hydrogen gas are both introduced into nitrobenzene?
A. 3- nitroaniline
B. 4 -nitroaniline
C. Aniline
D. 2 -hydroxyaniline
Answer: (C)
Solution: By substituting two oxygen atoms with two hydrogen atoms, the nitro (NO2) group of nitrobenzene is reduced to an amino (NH2) group, which is then converted to aniline or benzenamine.
Frequently Asked Questions (FAQs)
Q1. Is it possible to make amine from halides?
Answer: Alkyl halides can be converted into primary amines via a two-step process. Through an SN2 reaction with a cyanide anion, the alkyl halide is first transformed into a nitrile. The nitrile is subsequently changed into a primary amine by LiAlH4. Through each of these processes, an additional carbon atom is added.
Q2.. Can the Gabriel phthalimide synthesis be used to produce aromatic amines?
Answer: No. Because aryl halides can not undergo nucleophilic substitution with the salt produced by phthalimide, the Gabriel phthalimide synthesis cannot synthesize aromatic primary amines.
Q3. Where can I find amine?
Answer: Amine functional groups are found in a wide variety of compounds, including natural and synthetic colours, polymers, vitamins, and medications like penicillin and codeine. DNA, amino acids, hormones, and neurotransmitters are just a few of the substances that contain them and are essential to life.
Q4. Are amine and amino identical terms?
Answer: An amino group is a functional group in chemistry that consists of one bond connecting a nitrogen atom to a hydrogen atom, an alkyl group, an aryl group, or a combination of these three groups. Chemical compounds with an amino group are known as amines.



