-
Call Now
1800-102-2727
Average atomic mass - Definition, examples, practice problems & FAQs
A premium car showroom in your city mainly sells two models, one model on-road price is Rs.3500k and the other model on-road prize is Rs.9500k. Can you calculate the average car price sold by the showroom in one financial year?
At first look, it seems very simple that by calculating the arithmetic mean of the price of both models of cars. The average price is Rs.6500000 (), but here we made a wrong conclusion.
To find the average price of a car sold in a financial year, we must consider the sale percentage of the car (Model wise).
Similarly, elements also exist in different percentages in nature. So accordingly, like the scenario explained above, we must find the concerned element’s percentage of various forms in order to understand its behaviour more accurately.

Table of contents
- Average atomic mass
- Isotopes
- Percentage abundance
- Example
- Practice problems
- Frequently asked questions
Average atomic mass:
In the case of elements, a sample consists of more than one kind of atom called isotopes. Therefore, the Mass of a sample of atoms is also represented as weighted average mass and is called average atomic mass.
The weighted average of the atomic masses of an element's various naturally occurring isotopes is its atomic weight.
Isotopes:
Isotopes are particles with the same number of protons but different numbers of neutrons.
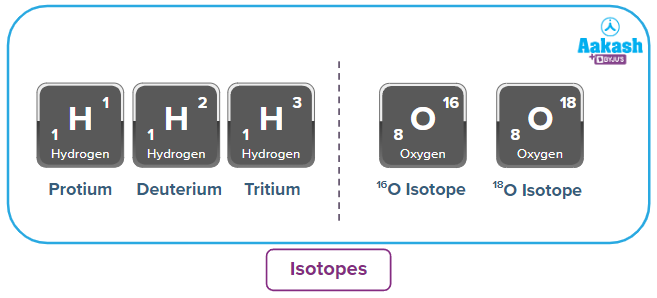
Percentage abundance:
Percentage abundance is defined as the percentage value of the number of isotopes available in nature for a given element.
So, an element's weighted average atomic mass is determined by multiplying the relative abundances of the element's isotopes by their atomic masses and then the summation of products.
average atomic mass =(% abundance)1 + (% abundance)1 Mass2 +………………
Average atomic mass =
Natural Abundances and Isotopic Masses Table
|
Name of element |
Sumbol |
Percentage Abundance |
|
Carbon |
98.93 |
|
|
1.07 |
||
|
Chlorine |
75.78 |
|
|
24.22 |
||
|
Copper |
69 |
|
|
31 |
||
|
Bromine |
51 |
|
|
49 |
Example 1.
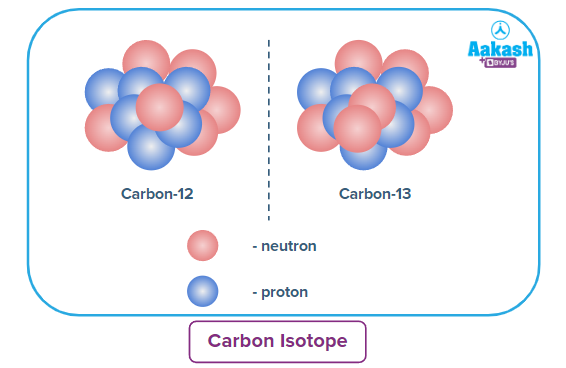
Abundance of Carbon-12 isotope → 99%
Abundance of Carbon-13 isotope → 1%
Average atomic mass of carbon =
Video link: Mole Concept Class 11 Chemistry Some Basic Concepts of Chemistry Concepts, Questions for JEE Mains
(Time: 12:19 to 14:39)
Practice problems
Q1. What is the average atomic mass of tungsten? The relative abundance of these isotopes in nature is provided below table
|
Name of element |
Symbol |
Percentage Abundance |
|
Tungsten |
0.12 |
|
|
26.50 |
||
|
14.31 |
||
|
30.64 |
||
|
28.43 |
A. 181.56 amu
B. 182.56 amu
C. 185.43 amu
D. 183.89 amu
Answer: (D)
Solution: Percentage abundance of tungsten is given
|
Symbol |
Percentage Abundance |
|
0.12 |
|
|
26.50 |
|
|
14.31 |
|
|
30.64 |
|
|
28.43 |
Average atomic mass =
Average atomic mass of chlorine =
Q2. What is the abundance percentage of if it contains two types of atoms having atomic masses of 35 amu and 37 amu. The average atomic mass of chlorine is 35.5 amu.
A. 25 %
B. 45%
C. 75 %
D. 85 %
Answer: (C)
Solution: Given, the average atomic mass of chlorine = 35.5 amu
Let, % abundance of
So, % abundance of
We know, Average atomic mass =
x=75%
We can write, as per given data
|
Name of element |
Sumbol |
Percentage Abundance |
|
Chlorine |
75 |
|
|
25 |
Q3. Given that the abundances of isotopes Fe54, Fe56 and Fe57 are 5%, 90%, and 5% respectively,calculate the average atomic mass of Fe
A. 54.78
B. 56.65
C. 55.95
D. 55.67
Answer: (C)
Solution: Given, abundances of isotopes, Fe54, Fe56 and Fe57 are 5%, 90% and 5%
Average atomic mass =
Average atomic mass of Fe =
Q4. Select correct statement, during the calculation of the weighted average atomic mass of elements
A. Numerical value of average atomic mass value closer to isotopes having less % abundance
B. Numerical value of average atomic mass value closer to isotopes having high % abundance
C. Both of these
D. None of these
Answer: (B)
Solution: Numerical value of average atomic mass value closer to isotopes having high % abundance
E.g- case 1:
Given, abundances of isotopes, Fe54, Fe56 and Fe57 are 5%, 90% and 5%
Average atomic mass =
Average atomic mass of Fe =
We can see, the average atomic mass of iron is closer to isotopes having highest abundance percent.
case 2:
Given, % of
% of
Average atomic mass =
Average atomic mass of chlorine =
We can see, the average atomic mass of iron is closer to isotopes having highest abundance percent.
Frequently asked questions-FAQs
Q1. What are the units for average atomic mass?
Answer: The average atomic mass is expressed in units of 'u' or 'amu.'
Where, u - unified mass
amu-atomic mass unit
Q2. Why is fractional atomic mass or molar mass indicated someplace if relative atomic mass cannot be a fraction? e.g-
Answer: written because of its isotopes, Cl present in nature in different isotopes in different natural abundance (%). So, due to their isotopes, they have fractional mass. We can call it weighted average atomic mass.
Given, % of
% of
Average atomic mass =
Average atomic mass of chlorine =
Q3. What is the distinction between atomic mass and atomic weight?
Answer: Atomic weight is the weighted average of isotopic abundance in nature, whereas atomic mass is used for specific isotopes. Because it is related to the number of protons and neutrons in the nucleus, atomic mass is always a whole number.
Q4. Can you write the name of any element which do not have any isotopes?
Answer: Name of elements that do have any isotopes are Thallium, Fluorine, Sodium, Aluminium, Phosphorous, scandium, etc



