-
Call Now
1800-102-2727
Oxidative Reactions Involving Peroxy Acid and Hydrogen Peroxide – Epoxidation of Alkenes, Hydroboration-Oxidation, Baeyer-Villiger Oxidation & Dakin Reaction
Chemical reactions are an integral part of innovation, research and development. Oxidation is a special kind of chemical reaction that is capable of incorporating oxygen atoms into a molecule, thereby enabling the generation of oxidised molecules that have various special features that are manipulated in various innovative technologies.
Especially when it comes to organic compounds, certain oxidative reactions, involving peroxy acids, are a special kind of reaction that has proved to be very significant in the formation of a special class of compounds called ‘epoxides’ that is gaining high speed popularity in the chemical industry. Epoxides are used to obtain derived polymers that can make great adhesives and practical surface coatings such as various concrete slab castings.

Let's find out more about the various oxidative reactions that enable us to obtain important chemical compounds!
TABLE OF CONTENTS
- Oxidative Reactions Using Hydrogen Peroxide and Peracids
- Epoxidation of Alkenes
- Hydroboration-Oxidation of Alkenes
- Hydroboration-Oxidation of Alkynes
- Baeyer-Villiger Oxidation
- Dakin Reaction
- Practice Problems
- Frequently Asked Questions-FAQs
Oxidative Reactions Using Hydrogen Peroxide and Peracids
Oxidation is the process of losing electrons by a chemical species and the corresponding rise in its oxidation state. Alternatively, oxidation results in the gain of oxygen atoms.
The simplest peroxide and a reactive oxygen species with an oxygen-oxygen single bond is hydrogen peroxide (H2O2). When exposed to light, it breaks down slowly, but when organic or reactive substances are present, it breaks down quickly.
- Since H2O2 has an oxidising property, it can directly interact with double bonds in large organic molecules to produce organic peroxides.
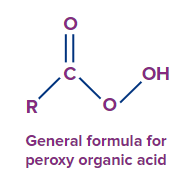
- Peroxy acids (RCO3H) are also stronger oxygen atom transfer agents and electrophilic peroxides. The acidity of the O-H bond correlates with the tendency of oxygen atom donation.
- CF3CO3H > CH3CO3H > H2O2 is the order of oxidising power. More electron-withdrawing substituents make the peroxycarboxylic acids more reactive.
- Alkaline hydrogen peroxide is used to oxidise alkylboranes into alcohols, which is the second step in the hydroboration-oxidation process.
- Besides that, peroxides and peroxy acids are also used in the epoxidation of alkenes, to epoxidize electron-deficient alkenes such as derivatives of acrylic acid.
- It serves as the key ingredient in the Dakin oxidation process as well as Baeyer-Villiger’s oxidation.
- Alcohols, ethers, esters, amides, and carboxylic acids are not easily oxidised by peracids.
Let’s see these oxidative reactions in detail.
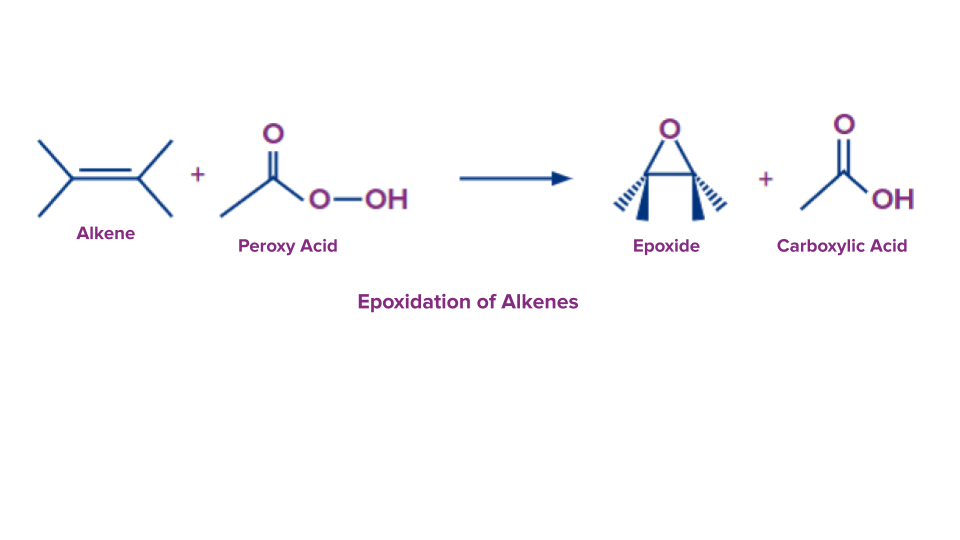
Epoxidation of Alkenes
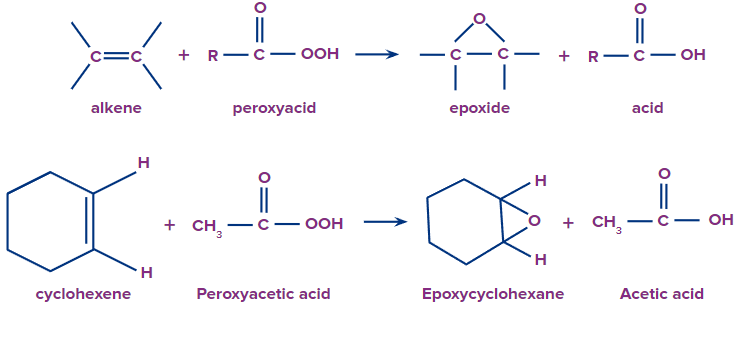
An oxygen atom is moved from the peracid to the C=C double bond and forms the oxirane ring during the epoxidation of alkenes. The chemical process known as epoxidation produces oxiranes from the carbon-carbon double bond (epoxides).
Peracids must be regarded as electrophilic oxidising agents since the transferred oxygen atom has a positive charge. In this procedure, peroxy acid (RCO3H) is used to transfer one oxygen atom.
The general reaction is as follows:
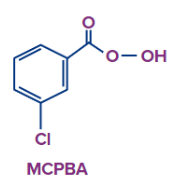
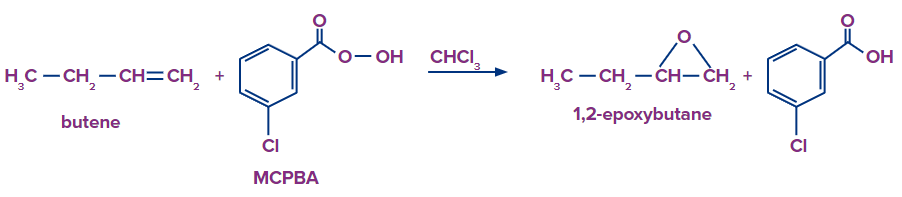
- The preferred epoxidation agent in laboratories is m-chloroperbenzoic acid (MCPBA). Because it stores well and is readily available in stores, m-chloroperbenzoic acid (MCPBA) is the most widely used peracid.
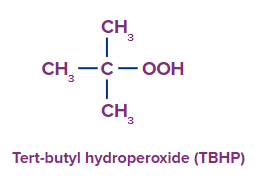
- Peroxybenzoic acid and peroxyacetic acid are two further peracids in use. The latter is produced on-site using hydrogen peroxide and acetic acid. Tert-butyl hydroperoxide (TBHP), dioxirane (CH2O2), and alkaline hydrogen peroxide are routinely utilised in addition to peracids.
- Let’s see some examples of Epoxidation: A peroxyacid is used in the epoxidation reaction to change an alkene into an epoxide. This reaction is basically an electrophilic addition of oxygen to the double bond of an alkene. Following are the examples:
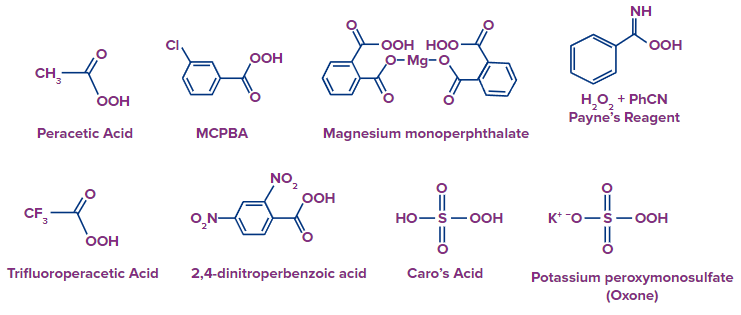
- Trifluoroperacetic acid and dinitroperbenzoic acid are electron deficient peracids that are needed for more intensely challenging oxidations (For example in Baeyer-Villiger oxidation of ketones, Trifluoroperacetic acid is used). Here are a few examples of peroxy acids:
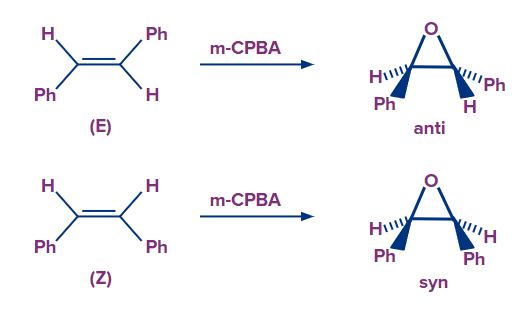
- Epoxidation reactions of alkenes are stereospecific i.e., the reaction permits the formation of one specific diastereomer over the other. Cis and/or trans epoxide diastereomers may develop depending on the mechanism of the reaction and the geometry of the alkene starting material.
Hydroboration-Oxidation of Alkenes
Alkenes can be changed into primary alcohols using the hydroboration oxidation reaction, and alkynes can be changed into ketones or aldehydes. A two-stage process that consists of an oxidation step and a hydroboration step is used to achieve this. This is done by adding water to the system net (across the entire double bond).
It is possible to think of the process of hydroboration-oxidation as an anti-Markovnikov addition of water molecule on alkene in which a hydroxyl group attaches to less substituted carbon.
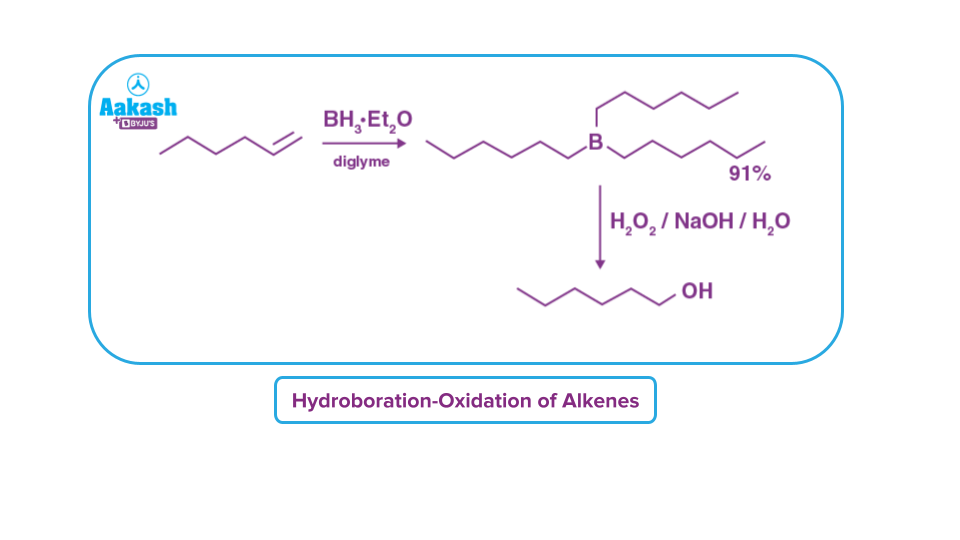
Hydroboration-Oxidation of Alkynes
Step 1: Hydroboration process
The alkynes can undergo hydroboration as per anti-Markovnikov rule. The boron atom goes to the least substituted carbon, which is also the least hindered, a top priority for the attack. 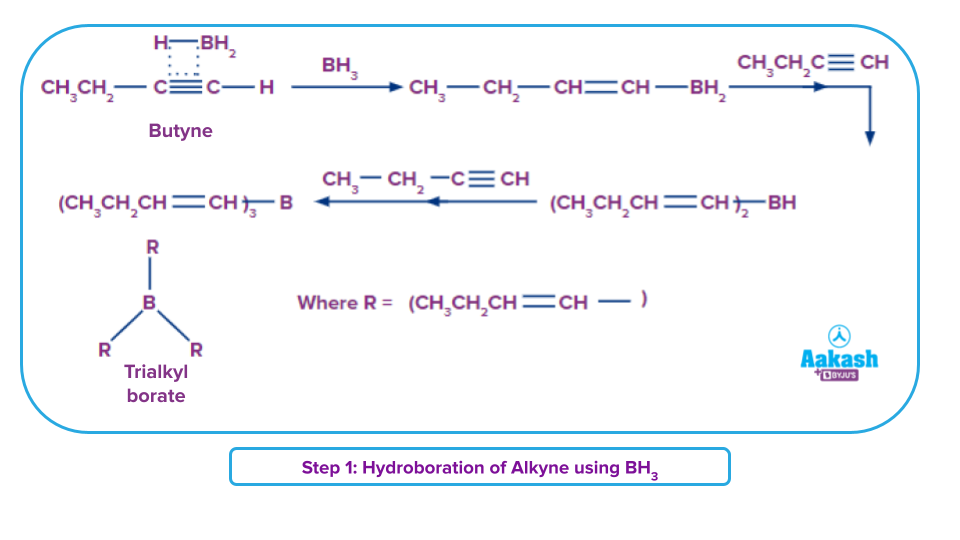
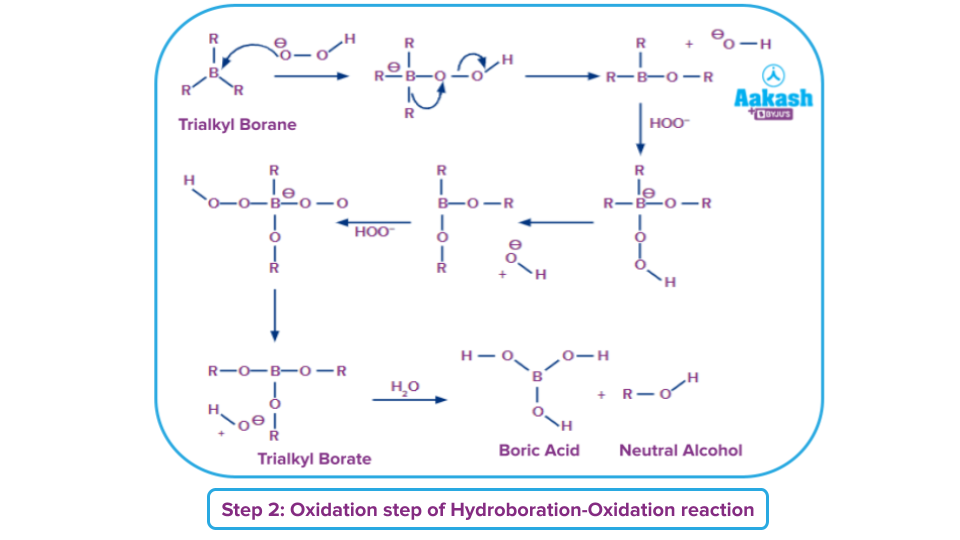
Step 2: Oxidation process
The second stage of the hydroboration procedure can now start since the trialkyl borane has been created. The hydroperoxide ion, a nucleophilic ion, attacks the boron atom in this step. The R group has now been repositioned along with its electron bond pair to the nearby oxygen atom.
Now that the hydroxide ion has been eliminated. Trialkyl borate is created by repeating this procedure three times. The necessary neutral alcohol is now created by treating this trialkyl borate with water. The following diagram shows how this mechanism phase works.
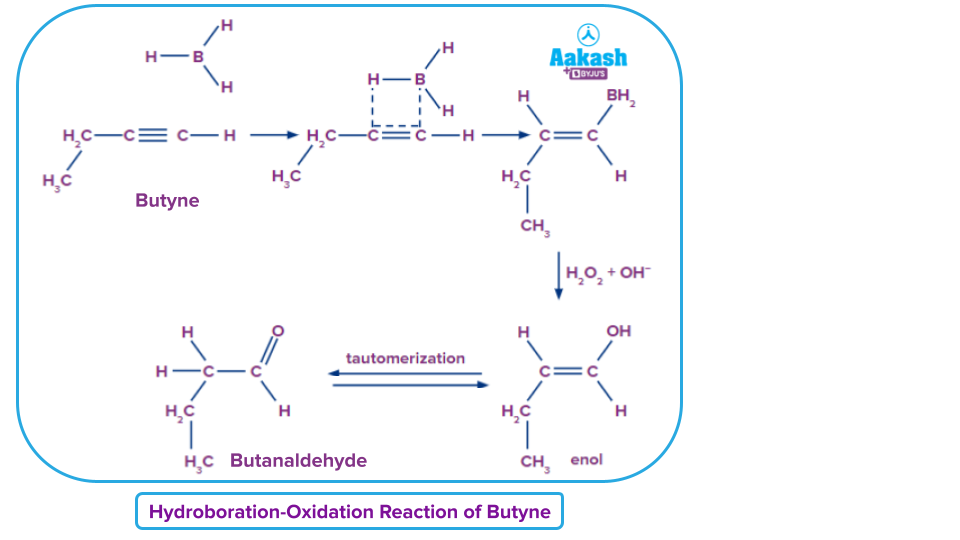
Example: Hydroboration-Oxidation of a Terminal Alkyne (Butyne)
Alkynes are transformed into ketone or aldehyde. The complete reaction can be divided into the following two phases. The first step is to add the borane on the 𝜋-bond of butyne and replaces all three hydrogen atoms. The second stage in this process is the conversion of the alkyl borane into vinyl alcohol, which has both an -OH group and an alkene group. The hydroxide reactions in the basic solution cause this oxidation to happen. Tautomerization is now used to this alcohol to create a stable aldehyde form.
Baeyer-Villiger Oxidation
By using peroxyacids or peroxides as the oxidant, the Baeyer-Villiger oxidation is a chemical reaction that converts a ketone into an ester and carboxylic acid or a cyclic ketone into a lactone or aldehydes to carboxylic acids. Adolf von Baeyer and Victor Villiger, who initially reported the reaction in 1899, are remembered by the reaction's name.
Aldehydes on treatment with peroxy acids produce a mixture of carboxylic acids. Ketones generate ester and carboxylic acids. This ‘O’ atom of the -OH group of a peroxy acid (RCO3H) gets inserted between the -H attached to the carbonyl carbon in an aldehyde, and the -R attached to the carbonyl carbon in a ketone.
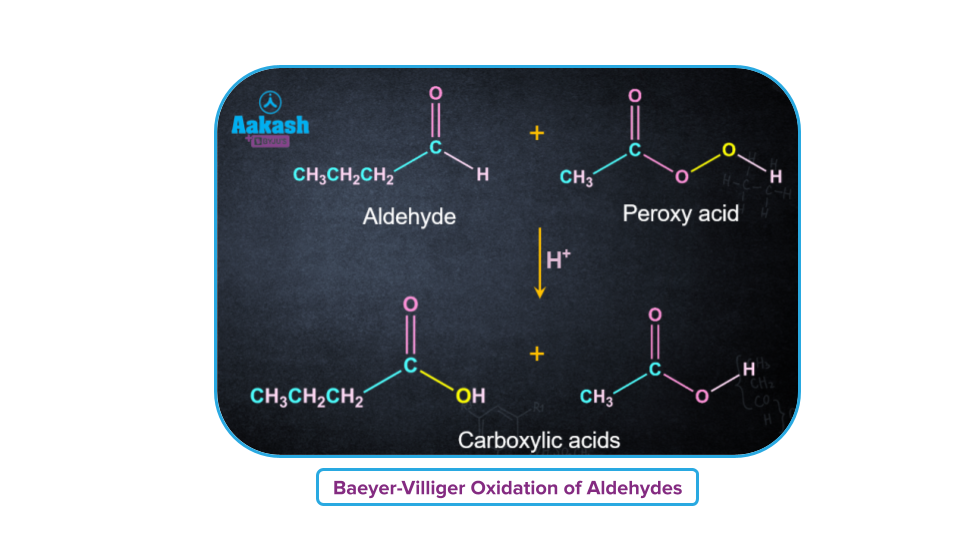
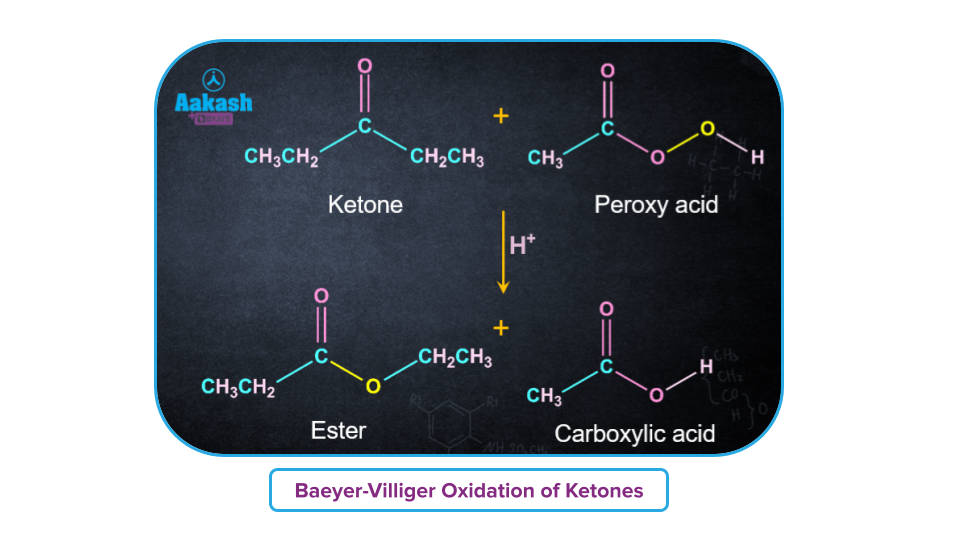
Mechanism of Baeyer-Villiger Reaction:
Peracids, such MCPBA, or hydrogen peroxide and a Lewis acid can be used in the Baeyer-Villiger.
Steps involved in this reaction are:
Step-1: Formation of a tetrahedral intermediate.
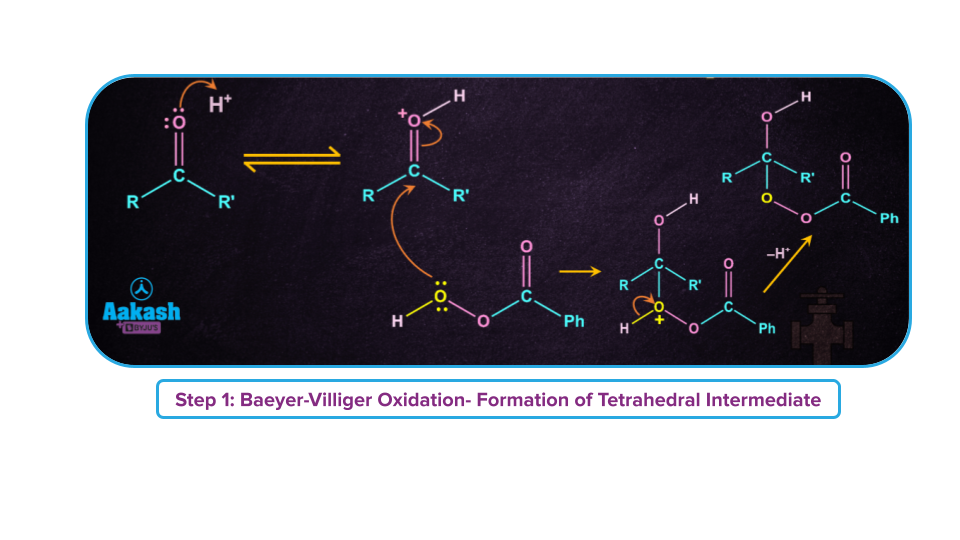
Step-2: Rearrangement of tetrahedral intermediate in a single step (concerted step) leading to the formation of ester and carboxylic acid.
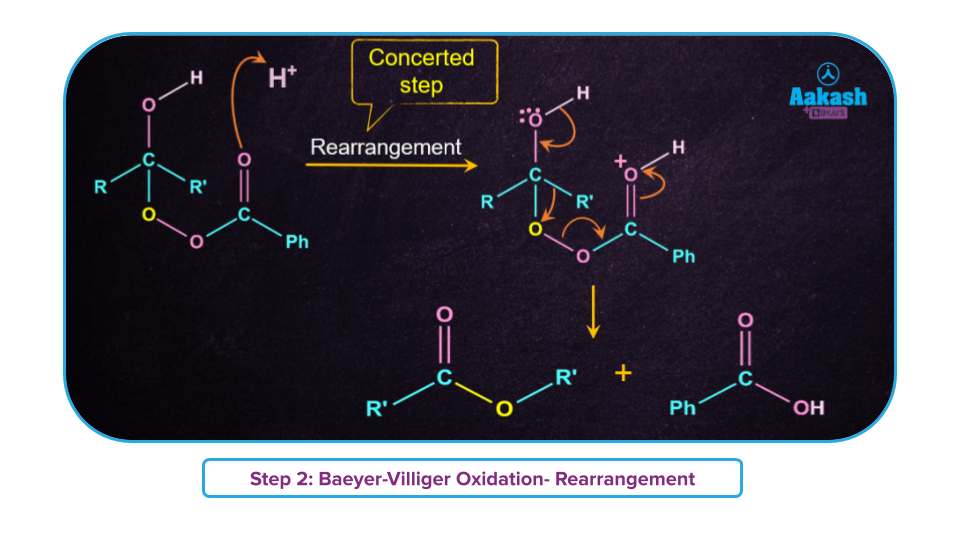
- If the ketone is unsymmetrical, a "O" insertion takes place between the carbonyl group and the -R group, which have a higher propensity for migration. For example for the tertiary alkyl group having more migratory power, "O" insertion takes place between the carbonyl group and the tertiary alkyl group.
- The following is the order of migratory aptitude in Baeyer-Villiger oxidation:
H>3o>Cyclohexyl>2o>Ph>1o>CH3
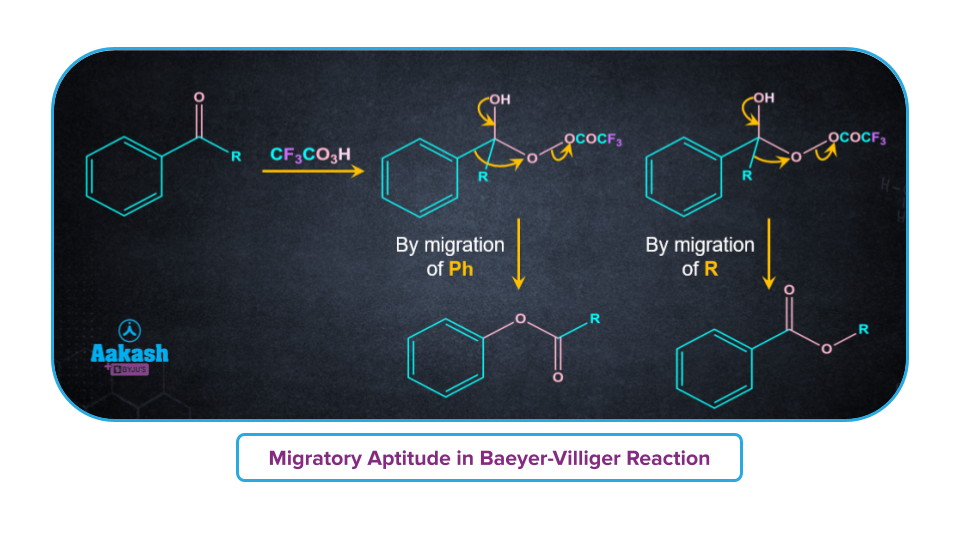
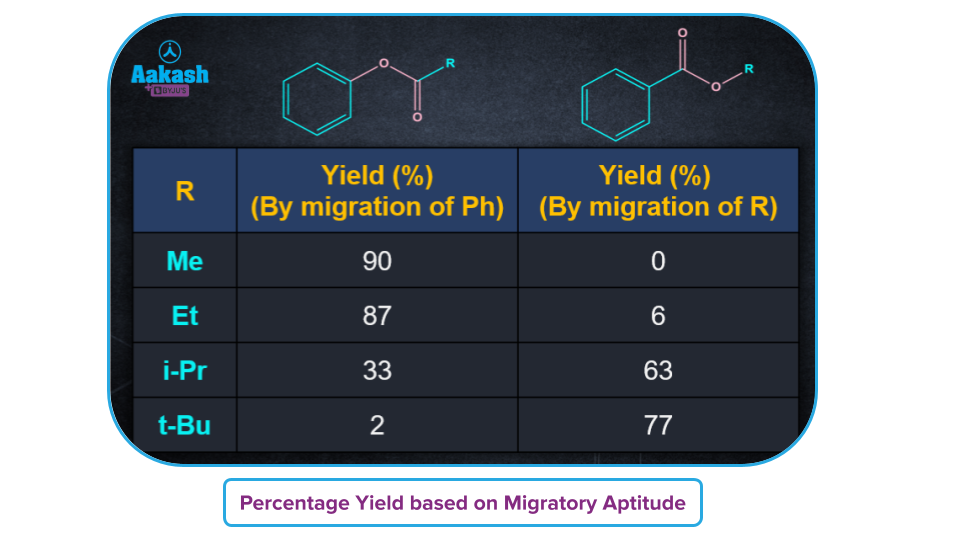
Dakin Reaction
An ortho or para hydroxylated phenyl aldehyde (2-hydroxybenzaldehyde or 4-hydroxybenzaldehyde) or ketone combines with hydrogen peroxide in the base to produce benzenediol and carboxylate ion in the Dakin oxidation (or Dakin reaction), an organic redox process. Overall, the hydrogen peroxide is reduced and the carbonyl group is oxidised.
The Dakin reaction enables the oxidation of aryl aldehydes or aryl ketones with hydrogen peroxide in the presence of a base to produce phenols.
The hydroperoxide anion is added nucleophilically to the carbonyl carbon to begin the Dakin oxidation, creating a tetrahedral intermediate (2). The intermediate collapses, resulting in the migration of [1,2]-aryls, the removal of hydroxide, and the creation of a phenyl ester (3). Following hydrolysis of the phenyl ester, a second tetrahedral intermediate (4) is formed by the nucleophilic addition of hydroxide from the solution to the ester carbonyl carbon.
This intermediate collapses, removing a phenoxide and creating a carboxylic acid (5). In the end, the phenoxide separates the acidic hydrogen from the carboxylic acid to produce the products that were gathered (6).
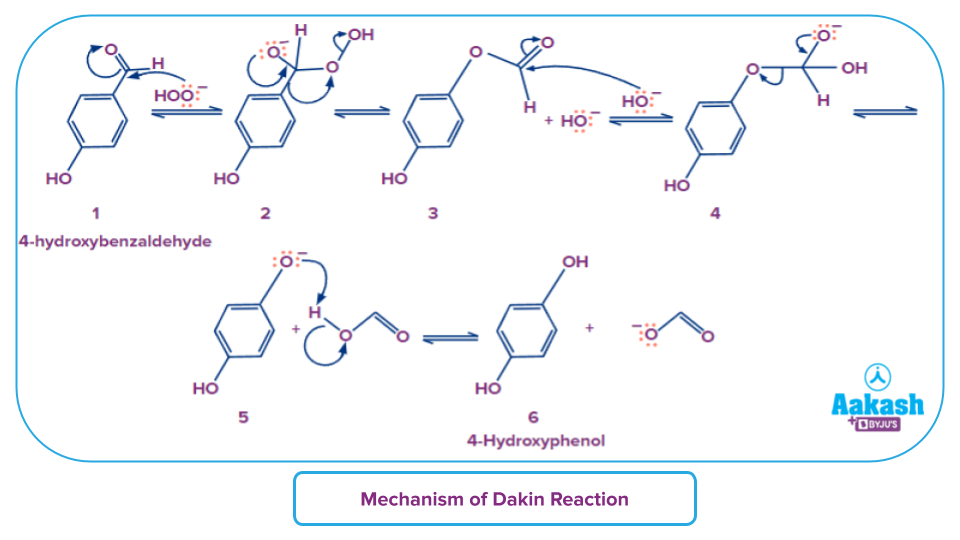
Practice Problems
Q.1. Epoxides are considered to be:
a. Electrophilic
b. Nucleophilic
c. Both
d. None of the above
Answer: (A)
Solution: The carbons in an epoxide group are extremely reactive electrophiles because a significant amount of ring strain is released when the ring opens in response to a nucleophilic attack. Typically, an alkene is oxidised in a laboratory setting to produce epoxides. Epoxides have a strained three-membered ring structure, where nucleophile attack and carbon release the ring strain, and this is what makes them electrophilic. So option A is the correct answer.
Q.2. Cyclohexene on reaction with peracetic acid provides:
a. CH3COOOH
b. Epoxycyclohexane
c. HCOOOH
d. C6H5COOOH
Answer: (A) & (B)
Solution: The formula of peroxy acetic acid is CH3COOOH and it is used in the preparation of epoxides. So on reaction with cyclohexene it undergoes epoxidation as follows.
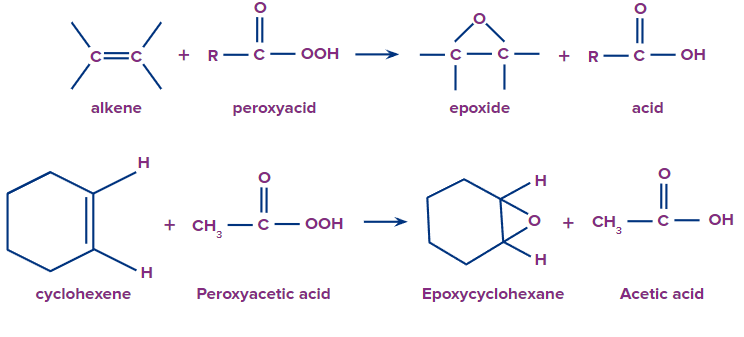
So, both options A and B are correct.
Q.3. What is the product obtained when cyclic ketone undergoes oxidation using peroxy acids?
Answer: Cyclic ketones undergo oxidation to produce lactones on treatment with peroxy acids. Here is an example of the formation of cyclohexanone to caprolactone.
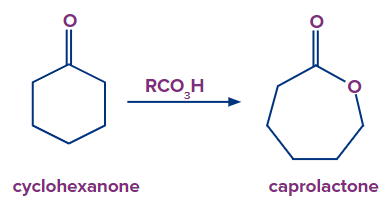
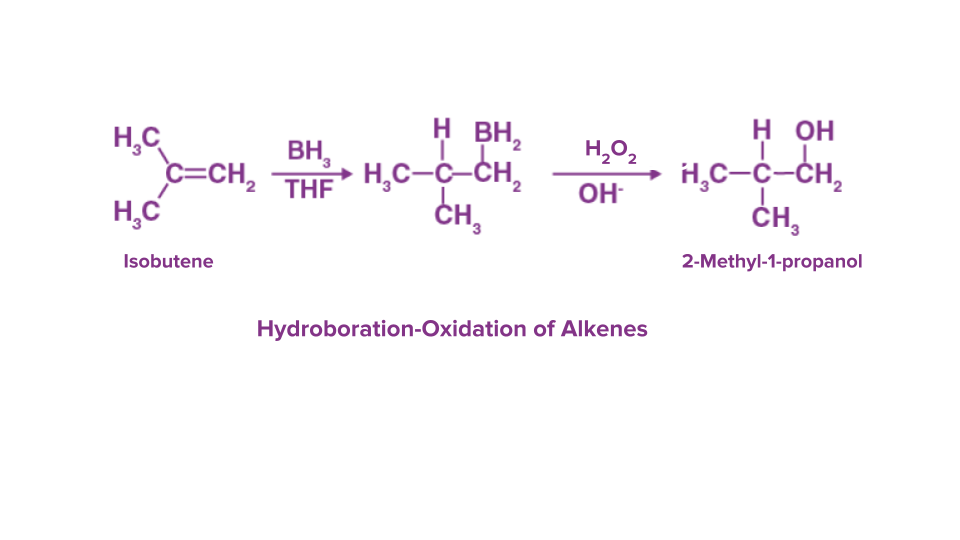
Q.4. In the hydroboration oxidation reaction, alkenes are oxidised to:
a. Aldehyde
b. Carboxylic Acids
c. Alcohols
d. Ketones
Answer: (C)
Solution: In organic chemistry, the hydroboration-oxidation process is a two-stage hydration reaction that converts an alkene into alcohol. Alkenes are transformed into neutral alcohols as a result. One mole of alkene needs three moles of BH3 to undergo hydroboration followed by oxidation using alkaline hydrogen peroxide.
Frequently Asked Questions-FAQs
Q.1. What sort of solvent is preferred for epoxidation of alkenes?
Answer: In either situation, a non-aqueous solvent is employed, such as ether, acetone, chloroform, or dioxane. The reason for this is that the epoxide ring hydrolyzes in an aqueous medium with any acidic or basic catalyst present to generate a vicinal diol, a molecule with two -OH groups on nearby carbons.
Q.2. What is the commercial use of epoxidation?
Answer: Epoxidation is one of the most frequently utilised reactions in organic chemistry due to its extensive applicability. Epoxides can be used as a starting point for the synthesis of 1,2-diols and numerous other 1,2-functionalized chemicals. Epoxides are used to create epoxy resins, a class of adhesives, most notably epichlorohydrin. Epoxides can be polymerized to create polyethylene glycols or polyoxyalkylene, which are utilised as waxes, detergents, lubricants, and parts of hydraulic fluid, depending on their molecular weight.
Q.3. What is ring strain?
Answer: Ring strain is a type of instability in cyclic organic molecules where carbon-carbon single bonds (carbons with sp3 hybridisation) form a ring structure. The carbon atoms in cycloalkanes are sp3 hybridised and should have the ideal 109.5o bond angle. But the actual bond angle in cycloalkanes is much less than this. For example the C-C-C bond angles in cyclopropane and cyclobutane are 60o and 90o, respectively. In cycloalkanes, the carbons are sp3 hybridised, which means that they do not have the predicted ideal bond angle of 109.5o. This leads to ring strain, which is brought on by the desire for the carbons to be at the ideal bond angle.
Hence they have internal angles that are significantly smaller than the idealised value. This generates ring strain and causes instability in such molecules.
Q.4. Is hydroboration-oxidation syn or anti-addition?
Answer: Here the approach is the addition of hydrogen and hydroxyl groups in the place where the double bond was present. Hydroboration-oxidation adds the less substituted carbon to the hydroxyl ring as per anti-Markovnikov rule. Hydroboration-oxidation is therefore a stereospecific (syn addition) reaction. In hydroboration-oxidation reaction hydrogen and hydroxyl group attacks the double bond form the same side, leading to cis stereochemistry (syn addition).



